High-yield Bioethanol Production from Cashew Apple in Atakpamé
Gnimdou Issanga Abli1,2 , Kosi Mawuéna Novidzro1,2*
, Kosi Mawuéna Novidzro1,2* , Komivi Akpo1,2 and Kossi Honoré Koumaglo1
, Komivi Akpo1,2 and Kossi Honoré Koumaglo1
1Laboratory of Process Engineering and Natural Resources (LAGEPREN), Université de Lomé, Togo.
2Department of Chemistry, Faculty of Science, Université de Lomé, Togo.
Corresponding Author E-mail:donnenovi@yahoo.fr/donnenovi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400627
Article Received on : 07 Oct 2024
Article Accepted on :
Article Published : 10 Dec 2024
Reviewed by: Dr. Malavika Bhattacharya
Second Review by: Dr.Siddaraju Mugur
Final Approval by: Dr. Abdelwahab Omri
The current work aimed to determine the efficiency of bioethanol production from cashew apples. To optimize bioethanol production yield, the juice extracted by mechanical pressing of cashew apples was concentrated into normal gravity (NG) must (20°Brix) and very high gravity (VHG) must (28°Brix). A parallel experiment was conducted using sucrose as a fermentable model substrate. Urea was added to enhance the fermentation capacity of the yeast. Batch and fed-batch fermentation processes were monitored by refractometric method, and the alcoholic content of the musts was determined using pycnometric method. The results showed that the ethanol produced rates (% vol.) with urea (2 g/L) in VHG must by the fed-batch process, and in VHG and NG musts by the batch process, were as follows: 16.36, 15.44, and 9.62, respectively. In comparison, ethanol obtained rates without urea were 15.37, 14.37, and 10.34 under the same conditions. Juice concentration, urea addition, and fed-batch fermentation process were used to optimize bioethanol production yields from cashew apples. In fact, the technology of bioethanol production adopted in the current study would generate additional benefits for farmers.
KEYWORDS:Additional benefits; Bioethanol; Cashew apple; Global warming; environmental protection
Download this article as:| Copy the following to cite this article: Abli G. I, Novidzro K. M, Akpo K, Koumaglo K. H. High-yield Bioethanol Production from Cashew Apple in Atakpamé. Orient J Chem 2024;40(6). |
| Copy the following to cite this URL: Abli G. I, Novidzro K. M, Akpo K, Koumaglo K. H. High-yield Bioethanol Production from Cashew Apple in Atakpamé. Orient J Chem 2024;40(6). Available from: https://bit.ly/3Zq6c8l |
Introduction
In recent years, the international community’s greatest concerns have been energy and environmental issues1. Indeed, the relentless rise in the price of petroleum products, the depletion of fossil fuel sources and the exacerbation of greenhouse gas (GHG) emissions are the main reasons for researchers to propose innovative alternatives able to reduce dependence on fossil fuels and protect the environment2-4.
To gain autonomy from pollutant energies, biofuels offer interesting prospects, as they are derived from inexhaustible sources5. In fact, biofuel technology is now recognized worldwide as a highly promising strategy for the substitution of fossil fuels in the future6,1. Consequently, there has been growing interest in recent years in discovering the appropriate mechanisms by which biofuels can be produced in sufficient quantities and without harm to the whole of humanity7. Indeed, rising petrofuel prices, geopolitical conflicts and permanent political instability in supply sites have made biofuel production highly competitive today8-10. Biofuel, by definition, refers to a liquid or gaseous fuel produced from plants, as distinct from petroleum-based fuel, whose combustion generates energy predestined for running vehicle engines and industrial machinery. Bioethanol and biodiesel are the main biofuels recognized worldwide due to the maturity of their production technologies11. Then, their use as credible alternatives is justified by the fact that their physicochemical and energy characteristics are very similar to those of conventional fuels. Nevertheless, their use in conventional engines is not without drawbacks. However, the presence of water in bioethanol or biodiesel can seriously cause dysfunctions in combustion engines originally designed for gasoline and diesel consumption respectively12,13. Due to the imminent decline in fossil fuels, several environmental problems and the global warming triggered by the use of petroleum resources 14,15, bioethanol is now becoming the best substitute for gasoline, the most consumed fuel in the transport sector16. However, one of the thorny issues undermining the development of the bioethanol sector, especially first-generation bioethanol, is its strong competition with human foodstuffs17,18. Added to this, there is also the limited ethanol tolerance of fermentative microorganisms and the highly diversified structural characteristics of the feedstocks available for use19. In fact, there are three fundamental types of feedstocks for bioethanol production: sugar substrates, starch products and lignocellulosic biomass20. At the current stage of bioethanol development, nothing is better than sugar feedstocks11. Feedstocks, being initially rich in fermentable sugars, enable higher bioethanol yields to be achieved with simple, quick-to-implement technologies. In contrast, starchy biomasses and especially lignocellulosic biomasses require complex technologies leading ultimately to very low bioethanol yields11,21. The main problem with starch and lignocellulosic biomass is that they cannot be directly fermented. Therefore, before they can be fermented into bioethanol, they must be processed through a depolymerization stage of starch, cellulose and hemicellulose, to produce fermentable monomeric sugars such as glucose1,22. Depending on the polymerization stage, these polymers require one or more pre-treatments to transform the complex polymer chains into simple sugars. This stage, which is often slow and energy-intensive, enables bioethanol to be produced, but the cost is still not competitive with petrofuels11,23,24. Today, bioethanol production is threatened by two serious obstacles. Firstly, it is causing famine due to the misappropriation of agricultural products and arable land to grow crops exclusively for energy purpose25,26. Secondly, conversion of non-food plant products, notably lignocellulosic biomass, into bioethanol requires significant resources, strategies and time, but regrettably leads to very poor bioethanol yields27. So, the question arises as to how best to overcome these two obstacles28.
In short, bioethanol production with good yields is hampered by non-optional exploitation of food crops intended for human consumption. To avoid competition between bioenergy and food, the use of non-edible agricultural products, rich in fermentable sugars, appears to be one of the most promising alternatives for bioethanol production.
In Togo, cashew crop is a mainly grown in four regions: “Central Region, Savannas Region, Kara Region, and Plateaux Region”, for cashew kernel production, mainly for export. However, cashew kernel production generates a significant amount of cashew apples, which rot in the crops. Currently, there are no specialised industries in Togo for extracting cashew apple juice. As this fruit is highly perishable and there is almost no means of preservation, nearly all cashew apples rapidly degrade in the crops. Yet, the juice from cashew apples, which is rich in fermentable sugars, can be valorized into value-added products, particularly bioethanol. So, in order to avoid all the severe criticisms levelled at the use of food products for energy purposes, we are turning our attention cautiously to the use of agricultural residues, which are both rich in fermentable sugars and non-food, to better integrate bioethanol into the energy mix of our country, Togo.
This approach has a twofold advantage, in particular high-yield bioethanol production and the fight against hunger in Togo. With this the background, the current work deliberately focused on the development of innovative techniques to optimize the production of 1st generation bioethanol from cashew apple, which until now have been widely viewed as a waste product, often neglected in cashew crops in Togo. This will add value to the apples for the benefit of those involved in cashew nut crops in Togo.
Materials and Methods
Raw Material
To produce bioethanol, cashew apples used as the raw material (Fig.1) were harvested in February 2022 in Atakpamé, a city located about 162 km from Lomé, the capital town of Togo. After their arrival into the laboratory, the apples were washed, and packed in plastic bags and stored in a freezer set at -23°C to prevent any potential degradation before their subsequent processing.
 |
Figure 1: Photograph of cashew apples used as raw material |
Physicochemical characterizations of the raw material
√ Water and volatile matter content determination
For this characterization, 5 g of cashew apples contained in a Petri dish were heated in an oven at 103 ± 2ºC until all water and volatile matter have been removed. Water and volatile matter content (Vm) is determined by formula 129.
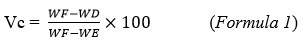
Where: WF = Weight of the fresh cashew apples + Petri dish before their introduction into the oven; MD = Weight of the dried cashew apples + Petri dish after their introduction into the oven; ME = Weight of the empty Petri dish; and Vm = Water and volatile matter content.
√ Dry matter rate measurement
Dry matter (Dm) is defined as the percentage by weight (%) of the dry matter of an organic substance in relation to its wet matter. In practice, this percentage was deduced from the determination of water and volatile matter (Vm) content, according to formula 229.

√ Ash content evaluation
Experimental protocol followed was to burn 5 g of cashew apples in a muffle furnace set at 550°C for two hours. The operation was carried out with a thermal gradient of 10°C/min. After destruction and total elimination of organic matter in inorganic form, made up of gases escaping from the crucible, the ash content (Ac) was calculated according to formula 329.
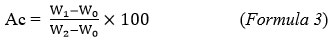
Where: Ac = Ash content of the cashew apples; Wo = Mass of the empty crucible; W2 = Total mass of the crucible and the sample before incineration and W1 = Total mass of the crucible and the ashes after incineration.
Pretreatment of the raw material
Cashew apples removed from the freezer were first washed, and then crushed by a mill. The crushed product is a mixture of liquid rich in fermentable sugars and fibrous residues. This mixture was first sieved, and the liquid collected was filtered by filter paper placed in a glass funnel. This mixture was first sieved and the recovered liquid filtered through filter paper inserted in a glass funnel. By heating without refluxing, the recovered filtrate, known as juice or raw must (13°Brix), was concentrated into two types of musts: normal gravity (NG) must (20°Brix) and very high gravity (VHG) must (28°Brix). After cooling to room temperature (30-32°C), these musts were carefully prepared and stored in a freezer (-23°C) for subsequent operations.
For comparison purposes, sucrose musts with concentrations identical to those of cashew apple musts (20°Brix and 28°Brix), were also prepared. Ethanolic fermentation of sucrose musts served as a model to be applied in the current work for bioethanol production from cashew apple. Before their usage, all previously prepared musts were first heated to 85°C for 20 min to eliminate all microbial flora, then cooled down to room temperature 30,31,32.
Ethanolic pre-fermentation
Pre-fermentation is the initial stage before proper ethanolic fermentation. At this stage, a fermentation inoculum was prepared with 1/10 each must (20°Brix or 28°Brix) volume, mixed with baker’s yeast of the genus Saccharomyces cerevisiae (1 g/L), and urea required as a growth factor, in a concentration range of 0-8 g/L. The resulting mixture was left to ferment anaerobically at room temperature (30-32°C) for 24 hours, to allow the fermenting microorganisms to acclimatize to their culture medium.
Start-up for ethanolic fermentation process
Each pre-fermented must was mixed with the remaining 9/10 volume of must waiting for the proper ethanolic fermentation. The pH was then adjusted to the optimum value of 4.533. The mixture was well stirred and left to ferment at room temperature (30-32°C). In practice, two ethanol fermentation processes were applied, specifically: batch and fed-batch fermentation.
Ethanolic fermentation control and alcoholic content analysis
The ethanolic fermentation reaction was monitored for thirty (30) days after the beginning of fermentation, by gaging total soluble matter (TSM), expressed in Brix degree, with Azzota Abbe refractometer, AR-2 model29. When the fermentation was accomplished, the final attenuation limit (FA) of each fermented must was calculated by formula 4.

The ethanol content in the musts at the end of fermentation was determined by pycnometric method as recommended by AOAC (Association of Analytical Chemists) 982.10.
Results
Physicochemical characteristics of cashew apples
Physicochemical parameters such as water and volatile matter content (Vc), dry matter rate (Dm), mineral matter or ash content (Ac), and organic matter (Om) rate of the cashew apple from Atakpame are presented in 2D pie chart (Fig. 2).
 |
Figure 2: Physicochemical characteristics of cashew apple from Atakpame |
The data displayed in this diagram indicated that cashew apple has a water and volatile matter content (Vc) of 71.17 ± 1.20%, while the dry matter rate or dryness (Dm) of 28.79 ± 1.16% corresponds to a mineral matter (or ash) content (Ac) of 2.76 ± 0.93% and an organic matter rate (Om) of 26.03 ± 0.33%.
Impacts of urea on ethanol fermentation of sucrose musts
√ Impact of urea on the evolution of soluble matter content in sucrose musts during batch ethanol fermentation
Evolution of total soluble matter (TSM) rate in NG an VHG sucrose musts during batch ethanolic fermentation process, as influenced by different urea concentrations, is shown in Fig. 3 and Fig. 4.
The relative positions of the curves presented in Fig. 3 and Fig. 4 illustrated the crucial role of urea in the efficiency of sucrose consumption, which is reflected TSM reduction in the musts.
 |
Figure 3: Impact of urea on TSM reduction during batch ethanolic fermentation process of NG sucrose must versus time |
 |
Figure 4: Impact of urea on the reduction of TSM during batch ethanolic fermentation process of VHG sucrose must versus time |
Impact of urea on ethanol rate produced by batch ethanolic fermentation of sucrose musts
The ethanol production rate (EPR: % vol.) by batch ethanolic fermentation process of NG sucrose musts and VHG sucrose musts, as a function of urea concentrations (0-8 g/L) was presented in Fig. 5. Ethanol rate values range between 8.47 ± 0.15% to 14.50 ± 0.24% (vol.) and 7.78 ± 0.14% to 15.16 ± 0.76% (vol.), respectively for NG sucrose musts and VHG sucrose musts.
 |
Figure 5: Impact of urea on ethanol rate (ER) produced by batch fermentation process of NG and VHG sucrose musts. |
Impact of urea on experimental yield of bioethanol production from batch fermentation process of sucrose musts
Experimental yields (EY) of bioethanol production in batch ethanolic fermentation process with NG and VHG sucrose musts, as a function of urea concentration, were recorded in Fig. 6.
 |
Figure 6: Impact of urea on EY of ethanol production by batch fermentation process of NG and VHG sucrose musts |
EY: Experimental yields
Impact of urea on the evolution of sucrose must concentration during fed-batch fermentation process
Fig. 7 shows the profiles of curves illustrating the variation in TSM content of sucrose musts during ethanolic fermentation in fed-batch process, depending on urea concentration. There is a large disparity between the curves for unenriched musts compared to those containing urea.
 |
Figure 7: Impact of urea on TSM content reduction, during fed-batch fermentation process of NG and VHG sucrose must depending time |
TSM: Total soluble matter
Impact of urea on EPR by fed-batch fermentation process of sucrose musts
Table 1 plots the EPR for fed-batch fermentation process of sucrose must, as a function of urea concentration.
Table 1: Impact of urea on EPR from fed-batch fermentation of sucrose must
|
Urea (g/L) |
EPR (% vol.) of VHG sucrose must in fed-batch fermentation process |
|
0.00 |
07.60 ± 0.30 |
|
2.00 |
16.48 ± 0.24 |
|
4.00 |
15.18 ± 0.77 |
|
8.00 |
14.69 ± 0.39 |
EPR: Ethanol production rate
Impact of urea on the bioethanol production yield from fed-batch fermentation of sucrose musts
EY (%) of bioethanol production by fed-batch fermentation process of VHG sucrose must, depending urea concentration are recorded in Table 2. The maximum EY of 75.07 ± 1.11% was obtained with 2 g/L of urea.
Table 2: Impact of urea on EY of bioethanol production by fed-batch fermentation process of VHG sucrose must
|
Urea (g/L) |
EY (%) of bioethanol production by fed-batch fermentation process of VHG sucrose must |
|
0.00 |
34.59 ± 1.36 |
|
2.00 |
75.07 ± 1.11 |
|
4.00 |
69.13 ± 3.49 |
|
8.00 |
66.90 ± 1.79 |
EY: Experimental yield; VHG: Very high gravity
Performance comparison of the three fermentation methods of bioethanol production with sucrose
The results presented in Table 3 show that, the final attenuation (FA) values of ethanolic fermentation of NG sucrose musts are higher than those of VHG musts. The presence of urea in the musts had a beneficial effect on the FA values. In addition, fed-batch fermentation process achieved higher FA values than batch fermentation process of VHG sucrose must. Meanwhile, FA values of NG sucrose must, fermented by batch process, were significantly higher than those of VHG sucrose must. In contrast, FA values of NG sucrose must, fermented by batch process, were significantly higher than those corresponding to batch fermentation process of VHG sucrose must.
Table 3: Final attenuation (FA) of NG and VHG sucrose musts
|
|
Final Attenuation (%) of sucrose musts |
||
|
Urea (g/L |
NG/Batch process |
VHG /Batch process |
VHG /Fed-batch process |
|
0.00 |
26.00 ± 1.41 |
20.97 ± 0.66 |
23.50 ± 2.12 |
|
2.00 |
66.25 ± 1.77 |
51.79 ± 2.52 |
56.07 ± 1.51 |
|
4.00 |
68.75 ± 1.72 |
47.32 ± 1.27 |
52.00 ±2.83 |
|
8.00 |
61.50 ±2.12 |
43.77 ± 1.29 |
46.72 ±0.40 |
FA: Final attenuation; NG: Normal gravity; VHG: Very high gravity
Tables 4 and 5 compare EPR and EY of bioethanol production by the three fermentation methods with NG and VHG sucrose, respectively.
Table 4: Impact of urea on EPR by the three fermentation methods of bioethanol production
|
|
EPR (% vol.) with sucrose musts |
||
|
Urea (g/L) |
NG/Batch process |
VHG/Batch process |
VHG/Fed-batch process |
|
0.00 |
8.47 ± 0.12 |
7.78 ± 0.11 |
7.60 ± 0.30 |
|
2.00 |
14.50 ± 0.18 |
15.16 ± 0.86 |
16.48 ± 0.24 |
EPR: ethanol production rate
Table 5: Impact of urea on EY of ethanol production by the three fermentation methods with NG and VHG sucrose must
|
Urea (g/L) |
EY (%) of ethanol production with sucrose musts |
||
|
NG/Batch process |
VHG/Batch process |
VHG/Fed-batch process |
|
|
0.00 |
53.92 ± 0.74 |
35.43 ± 0.48 |
34.59 ± 1.36 |
|
2.00 |
92.43 ± 1.12 |
69.10 ± 3.90 |
75.07 ± 1.11 |
EY: Experimental yield
These results highlight the benefits of bioethanol production optimization with sucrose through urea addition at 2 g/L.
With these interesting results found in the current study with NG and VHG sucrose musts, we decided to implement the technique of adding urea (2 g/L) for bath and fed-batch fermentation processes of cashew apple musts.
Impacts of urea on the fermentation of cashew apple musts
Impact of urea on TSM content in NG and VHG cashew apple musts during batch fermentation process
The variation of TSM content in NG and VHG cashew apple musts during batch fermentation process is shown in Fig. 8 and Fig. 9.
 |
Figure 8: Impact of urea on the reduction of TSM during batch fermentation process of NG must of cashew apple versus time |
 |
Figure 9: Impact of urea on the reduction of TSM during batch fermentation process VHG must of cashew apple versus time |
The shape of the curves depicted in Fig. 8 and Fig. 9 indicates that there is no significant difference between the pure must and the must supplemented with 2 g/L of urea.
Impact of urea on EPR by batch fermentation process of cashew apple musts
EPR by batch fermentation process of NG and VHG musts of cashew apple, conferring to urea concentration, is shown in Fig. 10. The results indicate that VHG must of cashew apple achieved the highest EPR compared to NG must of cashew apple. Although the addition of 2 g/L of urea led to an increase in EPR for the VHG must of cashew apple, in contrast, i.e., a decrease in EY of bioethanol production, was observed for NG must of cashew apple.
 |
Figure 10: Impact of Urea on EPR by batch fermentation process of NG and VHG musts of cashew apple |
Impact of urea on EY of bioethanol production by batch fermentation process of the musts of cashew apple
EY of bioethanol production by batch fermentation process of NG and VHG musts of cashew are exposed in Fig. 11.
 |
Figure 11: Impact of urea on EPY of bioethanol by batch fermentation of NG and VHG musts of cashew apple |
Urea addition with concentration of 2 g/L increased EPY by 4.88% with VHG must of cashew apple. However, with NG must of cashew apple, a decrease in EY of bioethanol production, approximately 4.67%, was detected.
Impact of urea on TSM content in cashew apple musts during fed-batch fermentation process
Fig. 12 illustrates TSM evolution in NG and VHG musts of cashew apple, during fed-batch fermentation process.
 |
Figure 12: Impact of urea on TSM content during fed-batch fermentation process of cashew apple musts versus time |
Relative positions of the two curves shown in Fig.12 indicate that in the latter stages of fermentation, the decrease in TMS in VHG must of cashew apple is greater than the case of NG must of cashew apple.
Comparison of the three fermentation methods of bioethanol production with cashew apple
Final attenuation comparison of fermented musts
The results presented in Table 6 specify that FA of VHG and NG musts of cashew apple with urea (2 g/L) addition are higher than unenriched musts across the three fermentation methods applied in the current work. However, fermentable sugar consumption by this fermentation process was less efficient compared to batch fermentation process of VHG must of cashew apple.
Table 6: FA of the musts of cashew apple, fermented by the three methods of bioethanol production
|
|
FA (%) of cashew apple musts |
||
|
Urea (g/L) |
NG /Batch process |
VHG/Batch process |
VHG/Fed-batch process |
|
0.00 |
53.60 ± 1.98 |
58.04 ± 1.27 |
50.32 ± 2.38 |
|
2.00 |
62.50 ± 3.54 |
61.56 ± 1.34 |
58.04 ± 3.78 |
FA: Final Attenuation
√ Impact of urea on EPR by fed-batch fermentation process of cashew apple must
Table 7 records EPR of bioethanol by fed-batch fermentation process of VHG cashew apple musts. Urea (2 g/L) usage increased EPR by approximately 0.99% (vol.). In large-scale production, this increase is beneficial for boosting the business’s financial viability.
Table 7: Impact of urea on EPR of bioethanol production by fed-batch fermentation process of VHG cashew apple must
|
Urea (g/L) |
EPR (% vol.) of bioethanol production of VHG cashew apple must |
|
0.00 |
15.37 ± 0.10 |
|
2.00 |
16.36 ± 0.24 |
√ Impact of urea on EY of bioethanol production by fed-batch fermentation process of VHG must of cashew apple
Table 8 displays the experimental yield of bioethanol production in batch fermentation process with NG and VHG musts of cashew apple. The results attest how 2 g/L of urea addition increased EY of bioethanol production by fed-batch fermentation process by around 4.48% with VHG cashew must.
Table 8: Impact of urea on EY of bioethanol production by fed-batch fermentation process of VHG must of cashew apple
|
Urea (g/L) |
EY (%) of ethanol fermentation in fed-batch fermentation of VHG must of cashew apple |
|
0.00 |
70.01 ± 0.44 |
|
2.00 |
74.49 ± 1.10 |
EY: Experimental yields
Discussion
The percentage of water and volatile matter of cashew apples is around 71.17% of the fresh mass of harvested apples. This high humidity and proportion of fermentable sugars in the raw juice (13°Brix) of cashew apple can be detrimental to the preservation of the fruits for bioethanol production. Indeed, with the presence of water in very high quantities in cashew apples very rich in fermentable sugars, the rate of degradation of organic matter may be exponential during their preservation. Hence, there is a need to process them quickly on site close to the crop of cashew apple34,3529. Nevertheless, the mineral content or ash content (Ac) of cashew apple (2.76 ± 0.93%) is lower than that of pineapple peels (5%) processed into bioethanol by29. The presence of these minerals would be a powerful benefit for bioethanol production. Indeed, the presence of some mineral elements, such as Mg, Zn, K, Ca, Co, Cl, Cu, Fe, etc., has a positive impact on ethanol fermentation rates36,37.
In contrast, the organic matter content (26.03 ± 0.33%) of cashew apple is very low compared to that (81%) of pineapple peel 29. This rate reflects the fraction of plant biomass derived from the photosynthesis mechanism carried out by the cashew plant during the effective conversion of atmospheric CO2 into carbonaceous substances, including carbohydrates, convertible into bioethanol36. With its substantial organic matter content, the cashew apple emerges as a potential source for bioethanol production38. To give credibility to the bioethanol industry, two conditions are required: the first is a complete renunciation of edible feedstocks, and the second is the deployment of high-yield bioethanol technologies. If these two conditions are achieved, bioethanol industry will receive all support needed from the international community, especially political decision-makers as well as human rights activists, consumer associations, non-governmental organizations (NGOs), and funding agencies. Therefore, it is necessary to find blameless alternatives for bioethanol sector. For this reason, our strategy focuses on the use of agricultural residues which are potentially rich in fermentable sugars, in particular cashew apple, to produce bioethanol. In addition, in view of ensuring process efficiency, must enrichment with a growth factor such as urea, raw juice transformation (13°Brix) into higher-concentration musts (20° Brix and 28°Brix), and batch fermentation process modified to fed-batch technology, were the various techniques employed to optimize the ethanol production rate. Thus, to be sure of the efficiency of ethanolic fermentation reaction, a preliminary investigation was carried out in the current study on NG (20°Brix) and VHG (28°Brix) sucrose. The best ethanol yield (16.48 ± 0.24% vol.) was found by fed-batch ethanolic fermentation process of sucrose must (28°Brix) enriched with urea (2 g/L). However, this improved ethanol yield does not correlate with the best experimental yield of ethanol production in the current study. In fact, the best experimental yield of ethanol production (92.43 ± 1.12%) in this work was attained instead by batch ethanolic fermentation process with NG sucrose must (20°Brix) enriched with urea (2 g/L). In addition, the poorest performance was observed by fed-batch fermentation of VHG sucrose must (28°Brix) without urea, penalized by an ethanol production rate of 7.60 ± 0.30% (vol.), equivalent to an experimental yield of ethanol production of 34.59 ± 1.36%.
By extrapolation of the results implemented in this study with NG and VHG sucrose musts (20°Brix and 28°Brix) to cashew apple musts, three (03) strategies were developed for bioethanol production. The first one involved concentration of raw juice (13°Brix) extracted from cashew apple into two types of higher concentration musts, NG must (20°Brix) and VHG must (28°Brix). The second strategy adopted was ethanolic fermentation of unenriched cashew apple musts (20°Brix and 28°Brix) by batch and fed-batch processes. Finally, the third strategy was addition of urea (2 g/L) to the cashew musts.
Overall, fed-batch fermentation process of VHG must (28°Brix) of cashew apple with urea (2 g/L) addition of achieved the highest ethanol production rates, while fermentation of must without urea addition (20°Brix and 28°Brix) provided the lowest experimental yield of ethanol production. In fact, with both sucrose and cashew apple musts, the best experimental yield of bioethanol production was always reached with fed-batch fermentation process by urea (2 g/L) adding. In contrast, fermentation pattern based on urea addition (2 g/L) into musts reduced experimental yield of ethanol production by around 4.67% with cashew apple must (20°Brix), fermented by batch fermentation process.
Bioethanol production method proposed in the current study, which consists by concentration of raw cashew apple juice (13°Brix) into concentrated musts (20°Brix and 28°Brix), offered advantage of optimizing ethanol production rate at the end of fermentation to around 15.44 ± 0. 32% (vol.) and 16.36 ± .24% (vol.), respectively in fed-batch with cashew apple musts (28°Brix) without urea, and with urea (2 g/L) addition, compared to 10.34 ± 0.04% (vol.) in batch fermentation process without urea. Experimental bioethanol yields equivalent to these ethanol production rates are around: 70.01 ± 0.44%; 74.49 ± 1.10% and 65.99 ± 0.26%. In this work, final attenuation (FA) values for ethanolic fermentation of cashew apple musts studied NG (20°Brix) and VHG (28°Brix) were: 53.60 ± 1.98%- 62.50 ± 3.54% versus 20.97 ± 0.66%-68.75 ± 1.72%. These values are lower than those found (67.18%-78.75%) by39 during their work carried out on cashew apple musts. The difference between the two comparative studies is explained by the fact that these authors fermented raw cashew apple juice with a lower concentration (13°Brix) than our own musts (20°Brix and 28°Brix).
The higher the concentration of the must, the lower is final attenuation (FA) after the related ethanolic fermentation. Moreover, the results of this study also confirmed the same hypothesis, i.e. 26.00 ± 1.41%-68.75 ± 1.72% for sucrose must (20°Brix) versus 20.97 ± 0.66%-56.07 ± 1.51%. In fact, final attenuation expresses the rate of fermentable sugars consumed during fermentation. Comparatively, ethanol rates produced with cashew apple in the current work were significantly higher, compared with the results found by 39,40 with cashew apple juice.
Conclusion
The various strategies developed in this work optimized ethanol production rate at the end of fermentation to 15.44 ± 0.32% (vol.) and 16.36 ± .24% (vol.), respectively by fed-batch fermentation process with VHG musts (28°Brix) of cashew apple without and with urea (2 g/L) addition, compared with 10.34 ± 0.04% (vol.) found with batch fermentation process without urea. These values are equivalent to experimental bioethanol yields of 70.01 ± 0.44%; 74.49 ± 1.10% and 65.99 ± 0.26%, respectively. Applying the method developed in this work on an industrial scale may generate additional benefits for cashew nut producers. However, our method still needs to be improved to enable actors in the cashew nut industry in Togo maximize their profits.
The next step in this work is to carry out further research to identify the most effective growth factors for optimizing the ethanol fermentation reaction using cashew apple juice.
Acknowledgement
Authors of this paper thank the authorities of the University of Lomé for their financial support, which allowed this study to be realized.
Conflicts of Interest
The author(s) do not have any conflict of interest.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Saratale, G. D.; Oh, S. E., Afr. J. Biotechnol., 2012, 11(5), 1002–1013.
- Sangdara, P.; Subsadsana, M.; Ruangviriyachai, C., Orient. J. Chem., 2017, 33(5), 2257–2262.
CrossRef - Awasthi, P.; Shrivastava, S.; Kharkwal, A. C.; Varma, A., Int. J. Curr. Microbiol. App. Sci., 2015, 4, 470–477.
- Laurent, P.; Roiz, J.; Wertz, J. L.; Richel, A.; Paquot, M., Biotechnol. Agron. Soc. Environ., 2011, 15, 597–610.
- Mignogna, D.; Szabó, M.; Ceci, P.; Avino, P., Sustainability, 2024, 16(16), 7036.
CrossRef - Holechek, J. L.; Geli, H. M. E.; Sawalhah, M. N.; Valdez, R., Sustainability (Switzerland), 2022, 14(8).
CrossRef - Levin, D. B.; Islam, R.; Cicek, N.; Sparling, R., Int. J. Hydrogen Energy, 2006, 31, 1496–1503.
CrossRef - Husain, S.; Sohag, K.; Wu, Y., J. Environ. Manage., 2024, 350, 119647.
CrossRef - Karkowska, R.; Urjasz, S., J. Commodity Markets, 2024, 36, 100440.
CrossRef - Wong, Y. C.; Lam, Z. K., Orient. J. Chem., 2019, 35(4), 1302–1312.
CrossRef - Aditiya, H. B.; Mahlia, T. M. I.; Chong, W. T.; Nur, H.; Sebayang, A. H., Renew. Sustain. Energy Rev., 2016, 66, 631–653.
CrossRef - Piri, H.; Renzi, M.; Bietresato, M., Energies, 2024, 17(1), 129.
CrossRef - Rimkus, A.; Pukalskas, S.; Mejeras, G.; Nagurnas, S., Sustainability, 2024, 16(6), 2397.
CrossRef - Balan, V.; Chiaramonti, D.; Kumar, S., Biofuels Bioprod. Biorefin., 2013, 7, 732–759.
CrossRef - Mood, S. M.; Golfeshan, A. H.; Tabatabaei, M.; Jouzani, G. S.; Najafi, G. H.; Gholami, M.; Ardjmand, M., Renew. Sustain. Energy Rev., 2013, 27, 77–93.
CrossRef - Novidzro, K. M., Éditions Universitaires Européennes, 2013, ISBN: 978-620-2-26274-3, 202 pages.
- Kouwanou, C. S.; Dossa Cokou, P. A.; Adjou, E. S.; Issiakou, M.; Wotto, V. D.; Sohounhloué, D. C. K., Acad. J. Chem., 2019, 4(10), 90–95.
CrossRef - Novidzro, K. M.; Melila, M.; Dotse, K., Koumaglo, K. H., Int. J. Chem. Stud., 2019, 7(6), 784-794.
- Mitra, S.; Paliya, S.; Mandpe, A., in Shah, M. & Deka, D. (Eds.), Emerging sustainable technologies for biofuel production (Environmental Science and Engineering), Springer, Cham, 2024.
- Balat, M.; Balat, H., Appl. Energy, 2009, 86, 2273–2282.
CrossRef - Ontanee, A.; Klinpratoom, B.; Subsadsana, M.; Ruangviriyachai, C., Orient. J. Chem., 2017, 33(5), 2507–2517.
- Silva, T. A. L.; Varão, L. H. R.; Pasquini, D., in Thomas, S.; Hosur, M.; Pasquini, D.; Chirayil, C. J. (Eds.), Handbook of Biomass, Springer, Singapore, 2024.
- El Hage, M.; Louka, N.; Rezzoug, S.-A.; Maugard, T.; Sablé, S.; Koubaa, M.; Debs, E.; Maache-Rezzoug, Z., Energies, 2023, 16(13), 5052.
CrossRef - Bansod, S. P.; Makwana, K.; Sarangi, P. K.; Parikh, J. K., Adv. Pretreatment Processes for Lignocellulosic Biomass to Biofuels Production, 2024.
CrossRef - Jambo, S. A.; Abdulla, R.; Mohd Azhar, S. H.; Marbawi, H.; Gansau, J. A.; Ravindra, P., Renew. Sustain. Energy Rev., 2016, 65, 756–769.
CrossRef - Binod, P.; Sindhu, R.; Singhania, R. R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R. K.; Pandey, A., Bioresour. Technol., 2010, 101(13), 4767–4774.
CrossRef - Ridley, C. E.; Clark, C. M.; Leduc, S. D.; Bierwagen, B. G.; Lin, B. B.; Mehl, A.; Tobias, D. A., Environ. Sci. Technol., 2012, 46, 1309–1315.
CrossRef - Correia, B.; Matos, H. A.; Lopes, T. F.; Marques, S.; Gírio, F., Processes, 2024, 12(11), 2580.
CrossRef - Novidzro, K. M.; Anoumou, K.; Fagla, B. A.; Melila, M.; Dotse, K.; Koumaglo, K. H., Int. J. Green Herb. Chem., 2019, 8(4), 938–953.
- de Medeiros Dantas, J. M.; Gómez Cardozo, J. R.; Beigbeder, J.-B.; Lavoie, J.-M., Ind. Crops Prod., 2023, 198, 116662.
CrossRef - Diakabana, P.; Kobawila, S. C.; Massengo, V.; Louembe, D., Cameroon J. Exp. Biol., 2013, 9(1), 1–8.
CrossRef - Sabba, G.; Aboubakar; Njintang, Y. N.; Mbofung, C. M. F., J. Ren. Energies, 2018, 21(1), 1–10.
- Novidzro, K. M.; Fagla, B. A.; Houndji, B. S.; Melila, M.; Dotse, K.; Koumaglo, K. H., Am. J. Chem. Eng., 2019, 7(4), 102–112.
CrossRef - Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Thumthanaruk, B.; Shetty, K., J. Food Sci. Technol., 2018, 55(10), 3979–3990.
CrossRef - N’guessan, Y. D.; Akpa, E. E.; Samagaci, L.; Ouattara, H.; Akoa, E. E.; Ahonzo-Niamke, S. L., Vinegar Production Trial from Cashew Apple (Anacardium occidentale), 2020.
- Aidoo, R.; Kwofie, E. M.; Ngadi, M. O., ACS Food Sci. Technol., 2022, 2(7), 1051–1066.
CrossRef - Chibi, S.; Djamel, E. H., Rev. Agrobiologia, 2018, 8(1), 685–694.
- Jeyavishnu, K.; Thulasidharan, D.; Shereen, M. F., Food Bioprocess Technol., 2021, 14, 985–1012.
CrossRef - Gbohaida, V.; Mossi, I.; Adjou, E. S.; Agbangnan Dossa, C. P.; Wotto, V.; Avlessi, F.; Sohounhloue, D. C. K., J. Appl. Biosci., 2016, 101, 9643–9652.
- Prommajak, T.; Leksawasdi, N.; Rattanapanone, N., Chiang Mai J. Sci., 2019, 46(3), 469–480.

This work is licensed under a Creative Commons Attribution 4.0 International License.









