Comparative Study of Phytochemical Composition and in vitro Antioxidant Activities of Pseudocedrela kotschyi Leaf, Trunk and Root Barks
Kodjo Anoumou1,2 , Kosi Mawuéna Novidzro1,2,*
, Kosi Mawuéna Novidzro1,2,* , Essodjolon Prospère Kanabiya1,2, Mamatchi Melila1,3
, Essodjolon Prospère Kanabiya1,2, Mamatchi Melila1,3 and Kossi Honoré Koumaglo1
and Kossi Honoré Koumaglo1
1Laboratoire de Génie des Procédés et des Ressources Naturelles (LAGEPREN), Université de Lomé, Togo
2Département de Chimie, Faculté Des Sciences, Université de Lomé, Togo
3Département de Biochimie, Faculté Des Sciences, Université de Lomé, Togo
Corresponding Author E-mail: donnenovi@yahoo.fr; donnenovi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400618
Article Received on : 30 Sep 2024
Article Accepted on :
Article Published : 12 Dec 2024
Reviewed by: Dr. Auchgatla Srinivasulu
Second Review by: Dr. Magda Mocanu
Final Approval by: Dr. Tanay Pramanik
The current work aimed to compare phytochemical composition and antioxidant activities of leaf versus trunk and root barks of Pseudocedrela kotschyi to discover whether the leave can be used effectively in traditional medicine instead of trunk and root in order to combat the extinction of the plant. Leave, trunk and root barks of P. kotschyi were successively extracted by maceration with hexane, dichloromethane and ethanol. Qualitative phytochemical composition and phytophenol contents of three ethanolic extracts were investigated. Antiradical activities and reducing power of the ethanolic extracts were evaluated to appreciate their antioxidant properties. The findings revealed that the leave has almost the same phytochemical composition compared to trunk and root barks of P. kotschyi, but its phenolic compound contents are lower and related to its antioxidant activities. A probable increase in the concentration of leaf recipes can probably allow their effective exploitation instead of trunk and root.
KEYWORDS:Antioxidant Activities; Ethanolic Extracts; Pseudocedrela kotschyi; phenolic compounds; Traditional medicine
Download this article as:| Copy the following to cite this article: Anoumou K Novidzro K. M, Kanabiya E. P, Melila M, Koumaglo K. H. Comparative Study of Phytochemical Composition and in vitro Antioxidant Activities of Pseudocedrela kotschyi Leaf, Trunk and Root Barks. Orient J Chem 2024;40(6). |
| Copy the following to cite this URL: Anoumou K Novidzro K. M, Kanabiya E. P, Melila M, Koumaglo K. H. Comparative Study of Phytochemical Composition and in vitro Antioxidant Activities of Pseudocedrela kotschyi Leaf, Trunk and Root Barks. Orient J Chem 2024;40(6). Available from: https://bit.ly/3Dl98LQ |
Introduction
For a long time, medicinal plants have contributed significantly to the well-being of populations. They constitute an immense source for the discovery of new bioactive molecules to be used to treat many pathologies1.
Despite the countless studies already carried out on plants, they still remain the most interesting and essential source for the discovery of natural molecules with bioactive potential. For this reason, medicinal plants are the primary pool of molecules whose isolation has enabled the production of highly effective pharmaceutical products. In fact, it is estimated that around 25% of all medicines sold in pharmacies worldwide are obtained from plants2. It seems that most of the medicinal plants already recorded on the globe are found in tropical countries, particularly in Africa, with very impressive therapeutic virtues3. So far, the poor populations of Africa have managed to survive despite the severity of certain epidemic diseases, often thanks to the use of proven phytomedicines.
During their growth, the ability of plants to synthesize various molecules, some of which have therapeutic properties, is linked to the fact that they undergo biotic and abiotic stresses in their environment. Abiotic stress refers to unfavorable environmental conditions such as drought, flooding, extreme temperatures, soil salinity and nutrient deficiencies. These stresses can impact the ability of the plants to fully perform their vital functions4. However, biotic stress involves attacks by living organisms such as: birds, mammals, herbivores, arthropods, microorganisms and weeds which cause serious problems for plants. All these various stresses then trigger the synthesis of natural substances in plants to allow them to develop, multiply and survive, despite the attacks to which they are constantly exposed. This makes plants potential sources of storage of bioactive substances5 which are used by humans as active ingredients in the formulation of medicinal products. Among the substances usually synthesized by plants, there is a class of biomolecules known as phenolic compounds. This is a heterogeneous group of phytoconstituents, each containing at least one phenol function in its structure. Among the phenolic compounds, flavonoids and tannins are considered to be the most studied subclasses, due to their therapeutic virtues considered to be very useful to humans. The mammalian body is also stressed daily due to the formation of free radicals, i.e. highly reactive unstable compounds containing single electrons. Unfortunately, in order to stabilize themselves, these free radicals attack certain vital biological molecules by creating other free radicals. This triggers chain reactions that have the disadvantage of degrading numerous cellular constituents, notably: DNA, lipids and proteins6.
Indeed, clinical disorders such as: cancer, liver disease, diabetes, heart failure, arterial hypertension, atherosclerosis, Alzheimer’s disease, rheumatic arthritis, hypercholesterolemia, neurodegenerative diseases, aging, immunological disorders and chronic inflammation7,8 are the adverse consequences arising from the harmful effects of free radicals in humans.
Phenolic compounds synthesized by plants are therefore historically highly reputed for combating the harmful effects due to free radicals. In Togo, the treatment of diseases using plants is still the most accessible method for the population, especially those of predominantly rural origin. Indeed, the proportion of the Togolese population who treat themselves with medicinal plants is around 60-80%9,10.
It is no longer a secret that the majority of molecules with therapeutic properties are produced by plants. One such plant that can be used by humans to stop or slow down the harmful effects of stress is P. kotschyi. In fact, previous studies have revealed that the aqueous extract of root bark and the ethanolic extract of the leaves both have impressive antioxidant properties11,12.
Furthermore, it has been reported that P. kotschyi is widely used in non-conventional medicine thanks to its multiple biological activities, such as: anticancer activity13 antioxidant activity11,12 antidiarrheal activity12, antidiabetic activity14, antimicrobial activities15, hepatoprotective activity16, antimalarial activity, anti-inflammatory activity, analgesic activity, antibacterial activity, anthelmintic activity and antipyretic activity. This is confirmed through previous studies showing that the root is often used for the treatment of numerous pathologies17. All these properties are often attributed to the phytochemical groups contained in various plant organs. However, overuse of the trunk and especially the roots of the plant for therapeutic purposes could lead to its disappearance, as it is often the whole plant that is uprooted after recovering the roots.
In the interest of preserving the plant by using the leaves for health care instead of trunk and root, this work focused on the phytochemical composition and antioxidant activities of ethanolic extracts of P. kotschyi leave in comparison with trunk and root barks.
Materials and Methods
Plant Material
Leaves, trunk and root barks, used as raw materials to extract the phytoconstituents of P. kotschyi, were harvested during october 2021, in Haho district, not far from the city of Notse, Togo. The plant was identified using the following code “TOGO 15976” in the LBEV laboratory of the Faculty of Sciences in Université de Lomé, Togo.
Methods
Pretreatment of plant material
After harvesting, all plant material (leave and trunk and root barks) was brought back to the laboratory, then dried for about two weeks, at room temperature (30-32°C) and away from sunlight. Then, every dry plant material was crushed by a mill (Thomas Scientific Laboratory Model 4, USA), equipped with a sieve of 1 mm pore diameter. The powders obtained were re-dried before being stored in well-labeled bottles, then preserved for later use.
Extraction of phytoconstituents contained in plant material
The extraction method applied in the present study was maceration, carried out successively with three organic solvents of increasing polarity, which are: hexane, dichloromethane and ethanol (95% vol.). The solid-liquid ratio 1:10 (m/v) was considered. In practice, 40 g of each sample of dry vegetable powder were successively extracted with 400 mL of each solvent. Indeed, in the first step, each vegetable powder was first degreased by soaking in hexane for 72 hours and with manual stirring. In the second step, the residue recovered after degreasing was extracted with dichloromethane. Finally, in the third step, the new residue obtained after extraction with dichloromethane was extracted again with ethanol.
The ethanolic solution obtained for each powder sample was filtered using filter paper. The solvent contained in the filtrate was removed under vacuum using Büchi rotary evaporator system. Then, the dry ethanolic extract was collected in a tinted glass bottle before being stored in a freezer whose temperature was set at – 23°C.
Qualitative phytochemical characterizations of ethanolic extracts
The different groups of biomolecules contained in the ethanolic extracts of P. kotschyi were highlighted by the staining and/or precipitation tests. Thus, the alkaloids were sought by the reaction with Dragendorff reagent18; phenolic compounds and tannins, respectively based on the Stiasny reagent and the test with FeCl319; flavonoids, by reaction with cyanidins20; anthocyanins by the reaction with hydrochloric acid (HCl), followed by addition of ammoniac NH321; coumarins, by adding 2 drops of NaOH (10% : w/v) to the extract followed by water bath heating22, anthraquinones, with ammoniac NH3 (10%) 23; cardiac glycosides cardiac glycosides, used a mixture of chloroform CHCl3 and acetic anhydride HOOC-CO-COOH with concentrated sulfuric acid H2SO4 24; reducing sugars with Fehling reagent test19; saponins, using foam test19; and finally, sterols and triterpenes, by the Liebermann-Burchard reagent22.
Determination of phenolic compounds found in ethanolic extracts of P. kotschyi
Total phenol contents in extracts
Total phenol contents in extracts were determined by applying the colorimetric method using UV-Visible spectrophotometer and Folin-Ciocalteu reagent25.
According to the experimental protocol, a volume of 1,600 µL of aqueous solution of Na2CO3 (6%: m/v) was mixed with 2,000 µL of aqueous Folin Ciocalteu reagent (10%: v/v) in a test tube. The previous mixture was agitated with vortex and subsequently left to stand for 5 min. Then, 400 µL of each ethanolic extract (1 mg/mL) or gallic acid GA (0-300 µg/mL) was added. The final mixture was agitated with vortex, then incubated for 30 min at room temperature (28-30°C) and away from light.
The absorbance of the resulting samples was measured with METASH UV-5200PC spectrophotometer against the blank at the wavelength of 760 nm. The calibration curve (Fig. 1) was established with gallic acid (GA) used as a standard for a concentration range of 0-300 µg/mL. The total phenol contents of the samples thus analyzed were expressed in mg of GA equivalent (Eq) per g of dry extract DE (mg GA Eq /g DE).
Total flavonoid contents in extracts
The contents of total flavonoids in extracts were measured by the colorimetric method using UV-Visible spectrophotometer26.
Experimentally, 200 µL of each aqueous ethanolic extract (1 mg/mL) or ethanolic quercetin QC (0-800 µg/mL), used as a standard, was mixed in a test tube with 1,600 µL of distilled water. Then, 80 µL of aqueous AlCl3 (10%: m/v) and 120 µL of aqueous NaNO2 (5%: m/v) were successively added. The previously prepared solution was incubated for 6 min, then added with 1 mL of aqueous NaOH solution (1 M) before being stirred with a vortex. The absorbance of the final solution was measured with METASH UV-5200PC spectrophotometer at a wavelength of 510 nm, against the blank. The total flavonoid contents of the samples were deduced based on a calibration curve (Fig. 2) established with quercetin QC (0-800 µg/mL). The results were expressed in mg of QC equivalent (Eq) per g of dry extract DE (mg QC Eq /g DE).
Total hydrolyzable tannin contents in extracts
The contents of total hydrolyzable tannins in extracts were carried out by colorimetric dosage with ferric chloride using UV-Visible spectrophotometer27.
According to the experimental approach, 1 mL of each ethanolic extract (1 mg/mL) or ethanolic tannic acid (TA: 0-200 µg/mL) was introduced into 3.5 mL of FeCl3 (0.01 M) prepared with aqueous HCl solution (0.01 M). The preceding mixture was vigorously vortexed before reading its absorbance at the wavelength of 660 nm against the blank, using METASH UV-5200PC spectrophotometer.
The total hydrolysable tannin contents were quantified in mg of TA equivalent (Eq) per g of dry extract DE (mg TA Eq/g DE), from a calibration curve (Fig. 3) constructed by TA (0-200 µg/mL).
Total condensed tannin contents in extracts
Determination of the contents of condensed tannins or proanthocyanidins or total non-hydrolyzable tannins in the three ethanolic extracts of P. kotschyi was obtained using the colorimetric method using UV-Visible spectrophotometer28.
In practice, 50 µL of each ethanolic extract (1 mg/mL) or ethanolic catechin (1-400 µg/mL) was introduced into a test tube containing 1,500 µL of the ethanolic vanillin (4%: m/v). The mixture gotten was agited vigorously with vortex, and 750 µL of HCl was added. The final solution was incubated at room temperature (28-30°C). After 20 min, the absorbance of the solution was measured with METASH UV- 5200 PC UV-visible spectrophotometer at the wavelength of 550 nm.
A calibration curve (Fig. 4) was performed with catechin CT (0-400 µg/mL), and the total condensed tannin contents were expressed in mg of catechin (CT) equivalent (Eq) per g of dry extract DE: mg CT Eq/g DE.
 |
Figure 1: Calibration curve of GA |
 |
Figure 2: Calibration curve of QC |
 |
Figure 3: Calibration curve of TA |
 |
Figure 4: Calibration curve of CT |
AG = Gallic acid; QC = Quercetin; TA = Tannic acid; CT = Catechin.
Antioxidant activity evaluation
Two types of antioxidant activities were evaluated in the current work: the test carried out with DPPH• reagent, and FRAP test. These two tests are called respectively : antiradical activity and reducing power.
Antiradical activity of the extracts
The protocol used to evaluate antiradical activity of the ethanolic extracts is described as follows29.
100 μL of each ethanolic extract (0-80 µg/mL) or ethanolic GA (0-80 µg/mL) were added to a 3,000 μL of ethanolic DPPH• (0.4%: m/v).
After an incubation period of 10 min at (28-30°C) and away from light, the absorbance of the solution was measured at 517 nm with METASH UV- 5200 PC UV-visible spectrophotometer against the blank. The tests were carried out in triplicate and inhibition percentage was calculated (Formula 1) for each measurement.
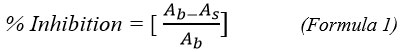
With: Ab: Absorbance of blank, and As: Absorbance of sample.
The average value of the three measurements carried out for each sample was taken into account. The linear curves of inhibition of the radical DPPH• by the extracts, and by gallic acid (GA) were plotted with a concentration range of 0-80 µg/ mL (Fig. 5).
The equations (Table 1) of the linear regression curves, established from Fig. 5, were used to determine the half-maximal inhibitory concentration (IC50) of the samples analyzed in the current study. The squared values of the correlation coefficient (R) of these curves denoted the precision of the analysis methods.
Table 1: Equations of linear regression curves for IC50 determination of the samples
|
Samples |
Equations |
R2 |
|
GA |
y = 1.1477 x |
0.9948 |
|
Le-Ext |
y = 0.5246 x |
0.9906 |
|
Tr-Ext |
y = 1.0377 x |
0.9952 |
|
Ro-Ext |
y = 0.8869 x |
0.9892 |
GA: Gallic acid; Le-Ext: Leaf extract; Tr-Ext: Trunk bark extract; Ro-Ext: Root bark extract; IC50: Half-maximal inhibitory concentration; and R: Correlation coefficient.
Reducing power of extracts
The method applied for reducing power evaluation of the extracts is described as below30.
FRAP reagent was prepared by mixing 160 mL of acetate buffer (300 mM, pH = 3.6), 20 mL of aqueous TPTZ-Fe3+ (10 mM) in HCl (40 mM), and 20 mL of aqueous FeCl3.6H2O (20 mM), in the proportions of (8:1:1). 2,000 μL of freshly prepared FRAP reagent was added to 1,000 μL of each ethanolic extract in ethanol (0-12.5 µg/mL) or ethanolic ascorbic acid AA(0-12.5 µg/mL).
The preceding mixture was incubated for 10 min, and the absorbance was measured at 593 nm with METASH UV-5200 PC UV-visible spectrophotometer against the blank.
The the extract reducing powers were evaluated from the established calibration curves (Fig. 6). An increase in absorbance corresponds to an increase in the reducing power of the extracts. Ascorbic acid AA was used as a reference.
The equations (Table 2) of the linear regression curves, established from Fig. 6, were used to deduce the reducing powers (RP) of the samples analyzed in the current study by using formula 2.
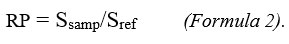
With: Ssamp: slope of the sample curve (expressed as mL/g); Sref: slope of the reference (AA) curve (expressed as mL/mg), and RP: Reducing power, expressed as mg Eq AA /g DE of each sample thus analyzed. The squared values of the correlation coefficient (R) of these curves indicated the precision level of the measurements.
Table 2: Equations of linear regression curves for RP determination of the samples
|
Samples |
Equations |
R2 |
|
AA |
y = 0.09281 x |
0.9996 |
|
Le-Ext |
y = 0.04692 x |
0.9940 |
|
Tr-Ext |
y = 0.07297 x |
0.9973 |
|
Ro-Ext |
y = 0.05214 x |
0.9991 |
AA: Ascorbic acid; Le-Ext: Leaf extract; Tr-Ext: Trunk bark extract; Ro-Ext: Root bark extract; RP: Reducing Power; and R: Correlation coefficient.
 |
Figure 5: Inhibition curves of DPPH radical by the samples |
 |
Figure 6: Calibration curves for RP of the samples |
GA: Gallic acid; AA: Ascorbic acid; Le-Ext: Leaf extract; Tr-Ext: Trunk bark extract; Ro-Ext: Root bark extract; RP: Reducing Power
Determination of correlations between phenolic compound contents and antioxidant activities highlighted with methanolic extracts
This correlation was established by plotting the variation curves of the phenolic compound contents as a function of its antiradical capacities evaluated by using DPPH reagent test (Fig. 7) and as a function of their reducing powers evaluated by using FRAP test (Fig. 8) according to the method applied in literature31, 32.
 |
Figure 7: Correlation between phenolic compound contents and antiradical capacities of the ethanolic extracts |
 |
Figure 8: Correlation between phenolic compound contents and reducing powers of the ethanolic extracts |
Results
Phytochemical composition of the ethanolic extracts
Phytochemical composition of the ethanolic extracts is recorded in Table 3.
Table 3: Phytochemical constituents detected in ethanolic extracts of P. kotschyi leaf, trunk bark and root.
|
Phytoconstituents revealed |
Le-Ext |
Tr-Ext |
Ro-Ext |
|
Alkaloids |
+ |
+ |
+ |
|
Phenolic compounds |
+ |
+ |
+ |
|
Flavonoids |
+ |
+ |
+ |
|
Tannins |
+ |
+ |
+ |
|
Anthocyanins |
– |
+ |
+ |
|
Coumarins |
+ |
+ |
+ |
|
Anthraquinones |
+ |
+ |
– |
|
Cardiac glycosides |
+ |
+ |
– |
|
Saponins |
+ |
+ |
+ |
|
Reducing sugars |
+ |
+ |
+ |
|
Triterpenes and sterols |
– |
– |
– |
Le-Ext: Leaf extract; Tr-Ext: Trunk bark extract; Ro-Ext: Root bark extract. (+): indicates the presence of phytochemicals; and (-): indicates the absence of phytochemicals.
Among the various phytoconstituents investigated, only triterpenes and sterols were not detected in the three ethanolic extracts of P. kotschyi. Anthraquinones and cardiac glycosides were only absent in the ethanolic root extract, while anthocyanins were only absent in the ethanolic leaf extract.
Phenolic compound contents in the three ethanolic extracts
Total phenol contents in the three ethanolic extracts
The results displayed in Fig. 9 show that the total phenolic contents (mg GA Eq/g DE) of the ethanolic extracts of leaf, trunk and root barks of P. kotschyi are: 261.732 ± 8.315; 383.3 ± 11.202; and 410.933 ± 4.220, respectively.
Total flavonoid contents in the extracts
The total flavonoid contents in the three P. kotschyi extracts are reported in Fig. 10. The results presented in Fig. 10 indicate that the leaf extract has a total flavonoid content of 477.417 ± 45.305 mg QC Eq/g DE, while those of trunk and root barks are 643.33 ± 79.605 and 729.583 ± 25.165 mg QC Eq/g DE, respectively.
Total hydrolyzable tannin contents in three ethanolic extracts
The total hydrolyzable tannin contents in the three ethanolic extracts of Pseudocedrela kotschy are recorded in Fig. 11. The values obtained (expressed in mg TA Eq/g DE) for the ethanolic extracts of the leaf, trunk and root barks are: 514.517 ± 62.61; 487.678 ± 26.637 and 720.413 ± 43.585, respectively.
Total condensed tannin contents in the three ethanolic extracts
In Fig. 12 are exposed the total condensed tannin contents in the three ethanolic extracts of Pseudocedrela kotschy. The values found (expressed in mg CT Eq/g DE) are: 133.170 ± 1.593; 638.671 ± 19.581 and 544.254 ± 10.931, respectively for ethanolic extracts in leaf, trunk and root barks of P. kotschy.
 |
Figure 9: TP contents of the ethanolic extracts |
 |
Figure 10: TF contents of the ethanolic extracts |
 |
Figure 11: THT contents of the ethanolic extracts |
 |
Figure 12: TCT contents of the ethanolic extracts |
TP: Total phenols; TF: Total flavonoids; THT: Total hydrolyzable tannins; TCT: Total condensed tannins; GA: Gallic acid; QC: Quercetin; TA: Tannic acid; CT: Catechin; DE: Dry Extract; Eq: Equivalent; Le-Ext: Leaf extract; Tr-Ext: Trunk bark extract; Ro-Ext: Root bark extract.
Antioxidant activities of the three ethanolic extracts
Antiradical activities of the three ethanolic extracts
The antiradical activities of the three ethanolic extracts of P. kotschy are illustrated in Fig.13. The concentration of dry extract required for 50% inhibition, noted IC50 (expressed in µg DE/mL) corresponding to the results of Fig. 13 are: 95.311 ± 1.126; 48.183 ± 1.521 and 56.372 ± 1.054, respectively for ethanolic extracts of leaf, trunk and root barks of P. kotschyi, while that of gallic acid, used as a reference, is 43.565 ± 0.970.
Reducing powers of the ethanolic extracts
The reducing powers (RP) of the ethanolic extracts of P. kotschyi, evaluated by means of reduction of ferric ion (Fe3+) to ferrous ion (Fe2+)ion test, allowed to obtain results reported in Fig. 14. The values found (expressed in mg AA Eq/g DE) are: 505.5489; 786.2299 and 561.7929, respectively for the ethanolic extracts of leaf, trunk and root barks of P. kotschyi.
 |
Figure 13: Antiradical capacities of the samples |
 |
Figure 14: Reducing powers of the samples |
Le-Ext: Leaf extract; Tr-Ext: Trunk bark extract; Ro-Ext: Root bark extract.; DE: Dry Extract; IC50: Half-maximal inhibitory concentration; Eq: Equivalent; AA: ascorbic acid; DE: Dry extract; AG: Gallic acid
Correlations between phenolic compound contents and antioxidant activities highlighted with methanolic extracts
The results obtained about the correlation between the phenolic compound contents and the antioxidant powers are summarized in Table 4.
Table 4: Correlation coefficients between phenolic compound contents and antioxidant capacities of ethanolic extracts
|
Antioxidants assays |
Correlation coefficients (R2) |
|||
|
TPC |
TFC |
THTC |
TCTC |
|
|
DPPH Assay |
0.8899 |
0.7646 |
0.0635 |
0.9998 |
|
FRAP Assay |
0.2636 |
0.1314 |
0.1804 |
0.6044 |
TPC: Total phenol content, TFC: Total flavonoid content, THTC: Total hydrolyzable tannin content; TCTC: Total condensed tannin content.
Strong correlations between the total phenol contents and the antiradical capacities of the three ethanolic extracts were observed according to the results presented in the table 2. However, there is no effective link between the total phenol contents and the reducing capacities of the extracts, except the total condensed tannin contents.
Discussion
The current work focuses on the effectiveness of exploitation in phytomedicine of the leaf instead of trunk and root barks of P. kotschyi in order to preserve this plant species, through the comparaison of phytochemical composition and the antioxidant activities of its three organs.
Phytochemical screening carried out on this plant simultaneously revealed the effective presence in the three ethanolic extracts of chemical groups such as: alkaloids, phenolic compounds, flavonoids, tannins, coumarins, saponins and reducing sugars (Table 3).
However, although anthocyanins were identified in the ethanolic extracts of trunk and root barks, on the other hand, they were absent in the leaf ethanolic extract. In addition, anthraquinones and cardiac glycosides were also reported in the leaf and trunk bark, but both were not found in the root bark of the plant. However, the search for triterpenes and sterols in all three ethanolic extracts of the plant was unsuccessful. The results presented in Table 3 are similar to those obtained for the leaf of this plant by 12; next for the bark of the trunk by33; finally for the root by34. Except, these authors did not report the absence of sterols and triterpenes in their works. 35firstly, and 36secondly, reported the presence of the same phytochemical compounds, respectively in trunk and the root barks, except alkaloids. This variability in the composition of secondary metabolites of plants could be related either to the period of harvest of the plant organs, or to the chemical composition or the pedological structures of the soils, or to the climatic factors, or to the stage of development of the plant35.
Based on the results found on the comparative phytochemical composition of the three ethanolic extracts of P. kotschyi, it can be deduced that, on the whole, the leaf contains almost the same phytoconstituents as trunk and root barks of the plant (Table 3). Therefore, the use of the leaf instead of the barks of the trunk and root in phytomedicine should probably lead to almost the same therapeutic efficacies. However, there is some enquiry about the degree of concentration of the phytoconstituents identically revealed in the leaf compared to the barks of the trunk and root. This aspect needs to investigate the contents of some phytoconstituents with therapeutic properties, in particular phenolic compounds.
The results of the current study revealed that root and trunk barks are respectively 1.57 times and 1.46 times richer in total phenolic compounds than the leaf.
Comparatively, these total phenolic compound contents found in the current work are all significantly higher than those found for the trunk bark of the plant by35, then38, which are respectively: 6.849 ± 0.326 mg GA Eq/g DE and 64.82 ± 0.99 mg GA Eq g DE. 37also found in the leaf of the plant a total phenolic compound content of 39.97 ± 3.63 mg GA Eq/g DE. This result was very lower than our own result. Contrary to this, the total phenol content found for the root bark in this work is lower than that found by11 which was 508.8 ± 4.5 mg GA Eq /g DE.
Concerning total flavonoids, the root bark also provided the highest content of 729.583 ± 25.165 mg QC Eq/g DE, and the leaf, the lowest content of 477.417 ± 45.305 mg QC Eq/g DE; while that of the trunk bark is 643.33 ± 79.605 mg QC Eq/g DE. Some authors11, 38, 39 have also reported in Burkina that total flavonoid contents of leaf, trunk and root barks were respectively: 4.86 ± 0.10; 3.00 ± 0.10 , and 10.4 ± 0.10 mg QC Eq/g DE. Nevertheless, all these values are very low compared to those found in the current work.
Regarding total hydrolyzable tannins, the highest content found in the current work was also recorded for the root bark (720.413 ± 42.585 mg TA Eq/g DE) while about total condensed tannins, it was rather trunk bark which had the high value (638.671 ± 19.581 mg CT Eq/g DE). However, the trunk bark has the lowest content of total hydrolyzable tannins (487.678 ± 26.637 mg TA Eq/g DE), followed by the leaf (514.517 ± 62.61 mg TA Eq/g DE), knowing that the contents of total condensed tannins (mg CT Eq/g DE) of the root bark and the leaf are respectively: 544.254 ± 10.931 and 133.170 ± 1.593.
In contrast, the total condensed tannin content of the trunk bark (130.977 ± 2.105 mg CT Eq/g DE) reported by33 was approximately 4.87 times lower than that obtained in this work.
It’s well known that the effectiveness of a plant organ in phytotherapy is directly linked to its biological activities among which the antioxidant activity occupies a significant place. For this reason, it is very important to evaluate antioxidant activities of ethanolic extracts leaf, trunk and root barks of P. kotschyi. However, since a single test is not enough to better classify the antioxidant powers of the compounds, then the three extracts of P. kotschyi were evaluated in the current work by two antioxidant methods. These include the radical method, measured with the DPPH• reagent, and the reducing power, evaluated by FRAP test.
Indeed, for the trapping of free radicals, the results found revealed that the trunk bark has the highest antiradical activity, with IC50 of 48.183 ± 1.521 µg DE/mL, while the leaf has the lowest antiradical activity, with IC50 of 95.311 ± 1.126 µg DE/mL, lower than that of the trunk whose IC50 is 56.372 ± 1.054 µg DE/mL. This ranking is explained by the fact that the antiradical power is inversely proportional to IC50 values. Therefore, gallic acid used as the reference molecule, with IC50 of 43.565 ± 0.970 µg DE/mL, stands out as the compound with the greatest antiradical activity compared to the three ethanolic extracts tested.
Regarding the values of reducing power of ethanolic extracts of P. kotschyi evaluated by FRAP test, trunk bark still shows itself as the best, with its reducing power of 786.2299 mg AA Eq/g DE ahead of the root bark and the leaf with their reducing powers of 561.7929 mg mg AA Eq/g DE and 505.5429 mg AA Eq/g DE, respectively.
Given the results found in the current work, it appears that ethanolic extract of trunk bark of P. kotschyi stands out as the most antioxidant while the ethanolic extract of the leaf is the least antioxidant, taking into account the results of the two antioxidant tests carried out.
In reality, the antioxidant capacity of a plant extract is generally proportional to its concentration of phenolic compounds. In this respect, since the leaf has the lowest contents of phenolic compounds (total phenols, total flavonoids, and total condensed tannins), consequently, this justifies its low antioxidant capacity. Similarly, if we only take into account the total condensed tannins, the fact that the ethanolic extract of the trunk bark is noted as the most antioxidant is quite logical. On the other hand, concerning the contents of total phenols, total flavonoids and total hydrolyzable tannins, we can believe that it is the ethanolic extract of the root bark that should be the most antioxidant but this was not verified in the current study.
In addition, the correlation between phenolic compound contents and antioxidant activities is also important. Correlation coefficients between 0.6 and 1.0 indicate a strong relationship31. In this context, previous studies have shown a strong correlation between phenolic compound contents and antioxidant activity40. In this work, the positive correlation results were obtained between total phenol, total flavonoid, total condensed tannin and total hydrolysable tannin contents and antiradical capacities of the three extracts. This correlation means that the antiradical capacity of the extracts mostly depends of the total phenolic compound contents. The antioxidant activities of the different extracts tested could be attributed to their richness in molecules with high anti-free radical potential such as polyphenols, flavonoids and especially condensed tannins. However, the correlation is moderately weak between the total phenol, the total flavonoid and the total hydrolyzable tannin contents and the reducing powers of the extracts. The disparity between the results of the two antioxidant tests applied in the current work should be justified by the fact that the mechanisms of action in which the phyto-organic compounds are involved vary according to the nature of the reactions involved but also the nature of the structures of these types of reagents.The antioxidant activities of the three extracts of P. kotschyi are due to the presence of flavonoids and especially condensed tannins. Thanks to phenolic compounds, the food and cosmetic industries have experienced a remarkable emergence41. Indeed, several studies have reported that polyphenols, including flavonoids and tannins, are endowed with very remarkable antioxidant activities, thus giving them biological properties such as anti-inflammatory; antitumor; antidiabetic; antihypertensive, and anticancer properties42. In addition, these phenolic compounds have positive effects in the treatment of cardiovascular and neurodegenerative diseases43, 44. They also have the particularity of inhibiting the peroxidation of lipid compounds, by acting as proton donors and free radical acceptors, thus stopping the auto-oxidation mechanism of fatty substances45, 46. The high antioxidant capacity of flavonoids and tannins is then explained by the existence in their intrinsic structures of several phenolic functions. Indeed, previous studies have shown that phenolic compounds containing trihydroxyl groups, such as gallotannins and galloylated proanthocyanidins, have a strong activity capable of neutralizing superoxide anions.O₂•- 42. For example, gallocatechins have the property of neutralizing HO• and HOO• radicals due to their redox potential. These free radicals are known as major contributors to several clinical disorders such as cancer, liver diseases, diabetes47, 48.
Conclusion
At the end of current study, we noted that the leaf of P. kotschyi contains almost the same secondary metabolites as the barks of the trunk and root, but its contents in total phenolic compounds are lower compared to those of the barks of the trunk and root. This attests to the results obtained for the antiradical activity and the antioxidant power of the leaf. The higher antioxidant activities of trunk and root barks compared to the leaf may justify their widespread use in traditional medicine in Togo. However, in order to obtain the same therapeutic results, it may be possible to increase the doses of the recipes formulated based on the leaf. Looking ahead, further studies on the leaf’s antimicrobial activities and toxicity are needed before promoting its use in herbal medicine.
Acknowledgement
The authors would like to thank the donors for their financial support for this project through PARESI program.
Conflicts of Interest
The authors declare that no conflict of interest exists regarding the publication of this paper.
References
- Saloufou, K. I.; Boyode, P. B.; Simalou, O.; Eloh, K.; Melila, M.; Kpegba, K.; Novidzro, K. M.; Gaslonde, T. et Michel, S. Int. J. Biol. Chem. Sci., 2017, 11(5), 2510-2520.
CrossRef - Serafini, M.; Rio, D. D.; N’Dri, D. Y.; Bettuzzi, S. and Peluso, I. 2nd edition, CRC Press/Taylor & Francis, 2011.
- Kolling, M.; Winkley, K. and von Deden, M. Global Health, 2010, 6(8), 1-9.
CrossRef - Chetto, O.; Dambier, D.; Fadli, A.; Benkirane, R.; Talha, A. et Benyahia, H. J. Appl. Biosci.,2015, 88,8154–8166.
CrossRef - Sarr, S. O.; Fall, A. D.; Gueye, R.; Diop, A.; Diatta, K.; Diop, N.; Ndiaye, B.; Diop, Y. M. Int. J. Biol. Chem. Sci., 2015, 9(3), 1263-1269.
CrossRef - Lobo, V.; Patil, A.; Phatak, A. and Chandra, N. Pharmacogn. Rev.,2010, 4,118-126.
CrossRef - Leong, L. P. and Shui, G. Food Chem., 2002, 76, 69-75.
CrossRef - Ramana, K.V.; Srivastava, S. and Singhal S. S. Oxid Med Cell Longev., 2013, Article ID 583438, 3 pages.
- Batawila, K. Mémoire DUEC ethnobotanique appliquée, Université de Lille, France, 2005, 53 pages.
- Sari, Contribution à l’étude des propriétés antifongiques de Elaeophorba grandifolia, Ficus polita, Microgyna inernis : trois espèces végétales au Togo, Thèse Doctorat, Université de Lomé, Togo, 2006, 55 papes.
- Sanou, Y.; Somda, M. B.; Ouattara, L.; Drabo, A. F.; Ouedraogo, R. J.; Meda, R. N. and Ouedraogo, G. A. J. Drug Delivery Ther., 2021, 11(6), 15-19.
CrossRef - Essiet, G. A.; Christian, A. G.; Ogbonna, A. D.; Uchenna, M. A.; Azubuike, E. J.; and Michael N. E. J. App. Pharm. Sci., 2016, 6 (03), 107-110
CrossRef - Kassim, O. O.; Copeland, R. L.; Kenguele, H. M.; Nekhai, S.; Ako-Nai, K. A. and Kanaan, Y. M. Anticancer Res.,2015, 35(3),1453–1458.
- Georgewill, U. O. and Georgewill, O. A. East. J. Med., 2019, 14(1),17–19.
- Ayo, R. G.; Audu, O. T.; Ndukwe, G. I. and Ogunshola, A. M. Afr. J. Biotechnol., 2010, 9, 7733–7737.
- Nchouwet, M. L.; Wansi Ngnokam, S. L.; Kodjio, N.; Poualeu, S. K.; Nkengeffouet, P. A. and Kamanyi, A. Asian J. Biomed. Pharmaceut. Sci., 2017, 7(63),1–9.
- Alhassan, A. M.; Ahmed, Q. U.; Malami, I. and Zakariad, Z. A. Pharm Biol., 2021, 59 (1), 953–961.
CrossRef - Jamal, S.; Barua, S.; Barua, A.; Morshed, A. J. M; Akter, R.; Akhter, S. Orient. J. Chem. 2024, 40(1), 74-81.
CrossRef - Devi, K. S; Singh, R. R; Thakur, L. K; Singh, T. P.; Singh, O. M. Orient. J. Chem. 2024, 40(4), 1159-1164.
CrossRef - Houta O.; Chouaeb H.; Neffati, M. and Amri, H. J. Soc. Chim. Tunisie, 2012, 14, 77-82.
- Senhaji, O; Faid, M; Elyachioui, M. et Dehhaoui, M. J. Mycol. Med., 2005, 15, 220-229
CrossRef - Karime, C. W.; Marcelline, A. N.; Anoubilé, B.; Baptiste, K. Y. M. J.; Thibaut, B. G.; Titah, J. T.; Faustin, K. A.; Daouda, B.; Claude, K. A. L. Orient. J. Chem. 2020, 36 (5), 804-811.
CrossRef - Rajbhar, K.; Dawda, H. and Mukundan, U. Sci. Revs. Chem. Commun., 2015, 5(1), 1-6
CrossRef - Himour, S.; Yahia, A; Belattar, H. et Bellebcir, L. JVB, 2016, 1(1), 34-38
- Gomathi, S. and Maneemegalai S. Orient. J. Chem. 2023, 39(3), 712-720
CrossRef - Mahmoudi, S.; Khali, M. et Mahmoudi, N. Nature & Technology, 2013, 9, 35-40
- Biaye, M. Thèse de Doctorat en pharmacie, Université Cheikh Anta Diop de Dakar, Sénégal, 2002, 53p.
- Ali-Rachedi, F.; Meraghni, S.; Touaibia, N. et Meshbah, S. Bull. Soc. r. sci. Liège, 2018, 87, 13 -21.
CrossRef - Kouamé, T. K.; Siaka, S.; Kassi, A. B. B. et Soro Y. Int. J. Biol. Chem. Sci., 2021, 15 (1), 97‑105
CrossRef - Kosanić, M.; Ristić, S.; Stanojković, T.; Manojlović, N. and Ranković, B. Farmacia, 2018, 66 (4), 644‑651.
CrossRef - Mbaiogaou A.; Madjitoloum Betoloum S.; Mbaihougadobe S.; Naitormbaide M.; Sale S.; and Mahmout Y. Int. J. Biol. Chem. Sci. 2022,16(4): 1475-1483
CrossRef - Bamba B.; Benie C. K. D.; Ouattara Abou; Doukourou D.N.; Kamou R. K. et ouattara K. Int. J. Biol. Chem. Sci. 2021, 15(1): 54-67
CrossRef - Alhassan, A. M.; Malami, I. and Abdullahi, M. I. Br. J. Psychol., 2014, 4(16),1937–1944.
CrossRef - Bothon, F. T. D.; Debiton, E; Avlessi, F; Forestier, C.; Teulade, J. and Sohounhloue, D. K. C. BMC Complement. Altern. Med., 2013, 13, 1–8.
CrossRef - Koudoro, Y. A.; Dedome, O.L. O.; Yehouenou, B.; Yovo M.; Agbangnan, D. C. P.; Tchobo, F.P.; Alitonou, G. A.; Akoegninou, A. and Sohounhloue K. D. Elixir Appl. Chem., 2014, 76, 28720-28726.
- Traoré, M. C. Thèse de Pharmacie, Faculté de Médecine, de Pharmacie et d’Odonto-Stomatologie, Université de Bamako, Mali. 2006, 176 pages.
- Manolaraki, F. Thèse de Doctorat, Université de Toulouse III-Paul Sabatier, Français; 2011, 185 pages.
- Delma, E. T.; Ouédraogo, M.; Ouédraogo, A. S.; Nikiema, A. W.; Gambo, M. A.; Ramde, N.; Youl, E. N. H.; Sanou, A. L.; Lompo, O. M. and Guissou, P. J. Exp. Pharmacol., 2023, 15, 231-240.
CrossRef - Sanou, Y.; Ouattara, L.; Kabre, P.; Ouedraogo R. J.; Ouoba, P.; Zante, A. A.; Zoungo, D.; Somda M. B. and Ouedraogo, A. G. J. Drug Deliv. Ther., 2023, 13(6),134-140.
CrossRef - Habouche, H. and Mimoune, S. . 2019, Université Mohamed Boudiaf de M’Sila. p. 133.
- Maroun, R. G. ; Karam, M. Spectra Analyse n° 295, 2013..
- Ayadi, S.; Dridi, N. et Ferhat Hamida, S. Mémoire de fin d’étude en Licence Académique de Biochimie, Université d’El-Oued, Algérie 2013, 45 pages.
- Badiaga, M. Thèse de doctorat en chimie organique, Université de Bamako. Autre. Université Blaise Pascal – Clermont-Ferrand II, 2011, Français, https://theses.hal.science/tel-00719564, 176 pages.
- Kumarappan, C. T.; Thilagam, E. and Mandal, S. C., Saudi J. Biol. Sci., 2012, 19(3), 349–355
CrossRef - Peronny, S. Thèse de Doctorat du Muséum national d’histoire naturelle, Discipline Eco-Ethologie. 2005, NNT. tel-0012546, 151 pages.
- Djemai, Z. S. Mémoire de Magister, Université el Hadj Lakhder -Batna, Algérie, 2009, 91 pages.
- Ngulde, S. I., Sandabe U. K. and Hussaini I. M. Afr. J. Pharm. Pharmacol., 2015, 9 (5),123-130
- Shinkafi, T.S.; Bello, L; Hassan, S.W. and Ali, S. J. Ethnopharmacol., 2015, 172 (22), 91-99.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









