New Equations Describing the Rheological Behavior of Chocolate
University of Bucharest, Faculty of Chemistry, Department of Physical Chemistry, Elisabeta Blvd, Bucharest, Romania.
Corresponding Author E-mail: istanciu75@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/400331
Article Received on : 15 Apr 2024
Article Accepted on : 01 Jun 2024
Article Published : 11 Jun 2024
Reviewed by: Dr. Mariana Domnica
Second Review by: Dr. Ericson Pereira
Final Approval by: Dr. Ayssar Nahle
The first archaeological mentions of cacao date back nearly 4,000 years and were discovered by researchers in Central America and Mexico. Chocolate is a compound that contains milk powder, cocoa, sugar, vanilla sugar and water.
The objective of this article is determination two rheological equations determined by the method of least squares and whose coefficients depend on the chocolate concentration and temperature. We used a DV-3P Anton Paar digital rotational viscometer.
Chocolate; Equation; Rheology; Temperature
Download this article as:| Copy the following to cite this article: Stanciu I. New Equations Describing the Rheological Behavior of Chocolate. Orient J Chem 2024;40(3). |
| Copy the following to cite this URL: Stanciu I. New Equations Describing the Rheological Behavior of Chocolate. Orient J Chem 2024;40(3). Available from: https://bit.ly/4ci4YRp |
Introduction
The first archaeological mentions of cacao date back nearly 4,000 years and were discovered by researchers in Central America and Mexico. Of course, it wasn’t about chocolate as we know it. For example, on the west coast of Mexico, archaeologists have discovered stone vessels dating to around 1900 before Christ. Before Christ, which contained residues of some cocoa drinks 1-5.
It was not until a thousand years later that the Olmec civilization began to cultivate the cocoa tree at a sustained rate. The Olmecs used cocoa beans and beverages made from these beans for medicinal purposes and in religious rituals. It is not known, however, how they prepared these drinks.
The first to leave written evidence of cocoa consumption were the Mayans, who even had a god dedicated to this tree in their pantheon. The cocoa drink was prepared as follows: the cocoa beans were left to ferment for several days. Once dried, the beans were roasted, ground, and the powder was then mixed with hot water and other ingredients such as hot pepper and oil.
Unlike the Mayans, the Aztecs consumed cold chocolate and believed it to have aphrodisiac properties and to give consumers energy and strength. The Aztecs were the first to use cacao beans as money: the Spanish conquistadors discovered that 100 cacao beans were the price of a canoe full of drinking water or a turkey.
The Aztecs were also the ones who gave the drink the name chocolate. Well, they called it “xocolatl”, from the words “xocol” – bitter and “atl”, which means water.
The first European to learn about chocolate was Christopher Columbus in 1502. On his fourth voyage to the Americas, Columbus captured a canoe with goods for trade that included cocoa beans. The explorer did not know what they were used for, and it was another 17 years before the conquistador Fernando Cortes observed the Aztec king Montezuma drinking cacao at his court in Tenochtitlan 5-11.
Spanish priests were the ones who first brought cocoa beans to Europe, and they were also the ones who introduced them to the court of the King of Spain, around the middle of the 16th century.
In the beginning, Europeans also consumed cocoa as a drink and even tried to recreate the Aztec recipes, with hot peppers and other spices. The Spanish believed cacao to have medicinal properties due to its bitter taste and used it to treat certain conditions such as abdominal pain.
It wasn’t until the early 17th century that Europeans began adding honey or other sweeteners to coffee, and the history of chocolate changed completely.
Today’s chocolate makers use mostly the same basic ingredients to make it: chocolate liquor, cocoa powder, cocoa butter, milk powder, sugar, lecithin, and flavorings or creams. The differences between the recipes are given by the specific ingredients for each producer and the amount of cocoa used. Each ingredient in chocolate has a well-established role: more milk powder makes the chocolate creamier, less sugar makes it more bitter – and more close to its natural taste.
After being harvested, the cocoa beans are fermented, then dried and ground.
Cocoa liquor is obtained from ground cocoa. It looks like a paste and does not contain alcohol. This paste has an intensely bitter taste and can be poured into moulds, where it solidifies and forms a raw chocolate.
Cocoa liquor is further processed and cocoa butter and cocoa powder are extracted from it. Both products are later used in chocolate making, but processing is necessary because it allows the composition to be changed for different chocolate recipes.
Cocoa powder consists of particles of fiber, protein and starch
Cocoa butter consists of vegetable fats with huge beneficial properties. It is the ingredient that gives chocolate the taste, color and texture we all know.
Cocoa butter is also the most expensive ingredient of chocolate, because it is also used in the cosmetics industry, for the manufacture of body lotions and creams.
The amount of sugar and milk powder added to the chocolate determines how sweet or bitter it is. White and milk chocolate contain the highest amount of sugar and are also among the most sought-after varieties of chocolate. The black or bitter one contains less sugar and is obviously bitter, but it is more suitable for cooking.
Another ingredient found in chocolate is lecithin, which everyone associates with its effect on memory. Lecithin is, however, a very good emulsifier, which reduces the viscosity of chocolate when melted and makes it easier to use.
Last, but not least, of the ingredients added to chocolate are the flavors and cream fillings. At the moment there are countless varieties of chocolate on the market, from the one with hot peppers to the dark chocolate lsa or the one filled with vanilla cream. It all depends on everyone’s taste. The rheological models found in the specialized literature do not completely describe the behavior of all fluids, including chocolate. That is why it was necessary to find new models that faithfully describe the rheological behavior of chocolate using the least squares method.
Consequently, in order to clarify the influences of various formulations of sugar substitutes on the rheological behaviour of compound milk chocolates, their shear stress vs. shear rate data was fitted with some reported mathematical models including power law (1), Bingham (2), Herschel-Bulkley (3), and Casson (4) equation 11-15:

where: σ – shear stress (Pa); κ – consistency coefficient (Pa.sn); γ – shear rate (s–1); μpl – plastic viscosity (Pa.s); σ0 – yield stress (Pa); K1 – Casson plastic viscosity (Pa.s); n – flow behaviour index (dimensionless). 15-23.
Material and methods
For the study of the rheological behavior, we used pure BIO chocolate (100% cocoa) that does not contain lecithin or traces of milk (Puratos Belcolade, Belgium). As the original chocolate sample, the one in the form of chocolate chips was used. Thus the chocolate samples were heated using a water bath with a digital controllable thermostat TC-650 (Brookfield, USA).
To determine the rheological behavior of chocolate, we used the Brookfield DV-III+ rheometer to measure the parameters of the sample.
This rheometer drives a spring into the sample and the deformation of the spring is measured with a transducer at t=36-42 °C and shear rates between 0.1s-1 and 68s-1.
Results and discussion
Figures 1 and 4 show chocolate rheogram (black curve) and polynomial regression (red curve) at temperature 36 °C. The studied chocolate has a non-Newtonian behavior in the temperature range at which it was studied.
 |
Figure 1: Chocolate rheogram (black curve) and polynomial regression (red curve) at a temperature of 36 °C |
Figures 2, 3 and 5 show chocolate rheogram (black curve) and exponential regression (red curve) at a temperatures 38, 40 and 42 °C. After fitting the experimental data obtained for chocolate, we found new rheological equations (5) and (6).
 |
Figure 2: Chocolate rheogram (black curve) and exponential regression (red curve) at a temperature of 38 °C. |
 |
Figure 3: Chocolate rheogram (black curve) and exponential regression (red curve) at a temperature of 40 °C |
Figure 4 show chocolate rheogram (black curve) and polynomial regression (red curve) at temperature 40 °C.
 |
Figure 4: Chocolate rheogram (black curve) and polynomial regression (red curve) t=42 °C |
 |
Figure 5: Chocolate rheogram (black curve) and exponential regression (red curve) at a t=42 °C |
Figure 6 shows the rheograms of the studied chocolate at temperatures between 36 and 44 °C.
 |
Figure 6: Chocolate rheogram at temperature B- 36°C, C- 38°C, D- 40°C, E- 42°C and F-44°C |
For regression, we determined coefficients of equation (5) for chocolate at a temperature of 36 °C.

where: σ – shear stress (Pa) and ÿ – shear rate (s–1).
Through polynomial regression, we determined the coefficients of equation (5) for chocolate at a temperature of 36 °C. The polynomial coefficients a and b are given in table 1.
Table 1: Values of coefficients for equation (5)
|
Temperature, °C |
Coefficients equations (5) |
r2 |
||
| σ0 |
a |
b |
||
|
36 |
12.29371 |
2.5251 |
-0.01664 |
0.99435 |
|
38 |
11.72995 |
2.18439 |
-0.01241 |
0.99452 |
|
40 |
11.65785 |
1.89279 |
-0.00775 |
0.99554 |
|
42 |
11.24701 |
1.77372 |
-0.00739 |
0.99529 |
|
44 |
11.02237 |
1.67435 |
-0.00716 |
0.99478 |
The value of the parameter σ0 is maximum at a temperature of 36 °C, after which it remains constant as we increase the temperature. The parameter decreases with increasing temperature. The same happens with parameter b. The correlation coefficients have values close to unity, which proves that the polynomial equation found faithfully describes the rheological behavior of the studied chocolate.
Through exponential regression, we determined the coefficients of equation (6) for chocolate at a temperature of 36 °C.
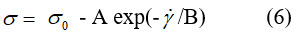
where: σ – shear stress (Pa) and ý – shear rate (s–1).
Table 2 includes the coefficients of equation (6) obtained by exponential regression the temperature 36 – 44 °C. The exponential coefficients A and B are given in table 2.
Table 2: Values of coefficients for the equation (6).
|
Temperature, °C |
Coefficients equations (6) |
r2 |
||
| σ0 |
A |
B |
||
|
36 |
143.7769 |
-131.77566 |
48.59911 |
0.99534 |
|
38 |
149.19144 |
-137.66725 |
59.65072 |
0.99527 |
|
40 |
179.8364 |
-168.37923 |
84.66939 |
0.99611 |
|
42 |
165.33904 |
-154.28669 |
82.63652 |
0.99589 |
|
44 |
151.3752 |
-140.54913 |
79.46577 |
0.99545 |
The coefficients of equations (5) and (6) were obtained by linear and exponential regression of the experimental data and depend on the chocolate concentration and temperature. The correlation coefficients in this case also have values close to unity. The values of the coefficients of the equations found using the least squares method depend on the chocolate concentration, i.e. the cocoa percentage, and on the temperature range at which the determination is made. At temperatures higher than 44 °C, the chocolate becomes fluid.
Conclusions
The article proposes two equations in which the coefficients were obtained by linear and exponential regression of the experimental data and depend on the chocolate concentration and temperature. The correlation coefficients have values close to unity.
Conflict of Interest
There is no conflict of interest.
References
- Gonçalves, E. V., & Lannes, S. C. D. S., 2010, Chocolate rheology. Food Science and Technology, 30, 845-851.
CrossRef - De Graef, V., Depypere, F., Minnaert, M., & Dewettinck, K., 2011. Chocolate yield stress as measured by oscillatory rheology. Food Research International, 44(9), 2660-2665.
CrossRef - LUENGO, Gustavo, et al. Thin film rheology and tribology of chocolate. Journal of food science, 1997, 62.4: 767-812.
CrossRef - Stanciu I. 2023. Some methods for determining the viscosity index of hydraulic oil, Indian Journal of Science & Technology, 16(4), 254-258
CrossRef - Stanciu I. 2023, Rheological behavior of corn oil at different viscosity and shear rate, Oriental Journal of Chemistry, 39(2), 335-339
CrossRef - Stanciu I. 2023, Rheological characteristics of corn oil used in biodegradable lubricant, Oriental Journal of Chemistry, 39(3), 592-595
CrossRef - Stanciu I. 2023, Effect of temperature on rheology of corn (Zea mays) oil, Oriental Journal of Chemistry, 39(4), 1068-1070
CrossRef - Chevalley, J., 1991. An adaptation of the Casson equation for the rheology of chocolate. Journal of texture studies, 22(2),219-229.
CrossRef - Taylor, J. E., Van Damme, I., Johns, M. L., Routh, A. F., & Wilson, D. I., 2009, Shear rheology of molten crumb chocolate. Journal of food science, 74(2), E55-E61.
CrossRef - Efraim, P., Marson, G. C., Jardim, D. C., Garcia, A. O., & Yotsuynagi, K., 2011, Influence of phytosterols addition in the rheology and sensory attributes of dark chocolate. Procedia Food Science, 1, 1633-1637.
CrossRef - Stanciu I., 2021, Oriental Journal of Chemistry, 37(1), 247-249
CrossRef - Stanciu I., 2021, Oriental Journal of Chemistry, 37(2), 440-443
CrossRef - Stanciu I., 2021, Oriental Journal of Chemistry, 37(4), 864-867.
CrossRef - Rohm, H., Böhme, B., & Skorka, J., 2018, The impact of grinding intensity on particle properties and rheology of dark chocolate. LWT, 92, 564-568.
CrossRef - Engmann, J., & Mackley, M. R., 2006, Semi-solid processing of chocolate and cocoa butter: modelling rheology and microstructure changes during extrusion. Food and Bioproducts Processing, 84(2), 102-108.
CrossRef - Götz, J., Balzer, H., & Hinrichs, R., 2005, Characterisation of the structure and flow behaviour of model chocolate systems by means of NMR and rheology. Applied Rheology, 15(2), 98-111.
CrossRef - Kiumarsi, M., Rafe, A., & Yeganehzad, S., 2017. Effect of different bulk sweeteners on the dynamic oscillatory and shear rheology of chocolate. Applied Rheology, 27(6), 11-19.
- van der Vaart, K., Depypere, F., Graef, V. D., Schall, P., Fall, A., Bonn, D., & Dewettinck, K., 2013, Dark chocolate’s compositional effects revealed by oscillatory rheology. European Food Research and Technology, 236(6), 931-942.
CrossRef - Stanciu I., 2019, Journal of Science and Arts, 3(48), 703-708.
- Stanciu I., 2019, Journal of Science and Arts, 4(49), 938-988.
CrossRef - Stanciu I., 2011, Journal of Science and Arts, 1, 55-58.
- Stanciu I., 2018, Journal of Science and Arts, 18(2), 453-458
- Pereira A., McInyre C., 2014, Appl. Rheol., 24(6), 11-19

This work is licensed under a Creative Commons Attribution 4.0 International License.










