Effect of Temperature Study on Low Carbon Steel in Hydrochloric Acid Medium using Novel Organic Inhibitor
Department of Chemistry, Padmavani Arts and Science College for women, Salem, Tamilnadu ,India.
Corresponding Author E-mail: singaravelup96@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400332
Article Received on : 15 Apr 2024
Article Accepted on : 29 May 2024
Article Published : 07 Jun 2024
Reviewed by: Dr. Loganathan S
Second Review by: Dr. Anand B
Final Approval by: Dr. Ioana Stanciu
Weight loss measurements and EIS-electrochemical impedance spectroscopy were applied to examine and compare the inhibitory effectiveness of piperazin derivative [4-(4-aminobenzoyl)-piperazin-1-yl]-(furan-2-yl) methanone (4-4-ABPFM) on the mild steel corrosion of in acidic media of 1N hydrochloric acid at different temperatures (303 and 313 K). Compound (4-4-ABPFM) exhibits mixed-type inhibitory behavior and suppresses both the cathodic and anodic processes, according to potentiodynamic polarization tests. It was discovered that the composition of the compounds under investigation mostly determined the inhibitory efficiency, and changes in impedance characteristics are suggestive of these compounds' adsorption on the metal surface. Additionally, these investigations have demonstrated the effectiveness of (4-4-APBFM) as an inhibitor on low carbon- MS in 1N HCl medium at two temperatures. The effectiveness of the inhibitor on mild steel is also revealed by surface morphological analyses like SEM.
KEYWORDS:(4-4-ABPFM); Anodic; Potentiodynamic Polarization; SEM
Download this article as:| Copy the following to cite this article: Singaravelu P. Effect of Temperature Study on Low Carbon Steel in Hydrochloric Acid Medium using Novel Organic Inhibitor. Orient J Chem 2024;40(3). |
| Copy the following to cite this URL: Singaravelu P. Effect of Temperature Study on Low Carbon Steel in Hydrochloric Acid Medium using Novel Organic Inhibitor. Orient J Chem 2024;40(3). Available from: https://bit.ly/3Kzy0jo |
Introduction
For mild steel, the reversal process of corrosion poses a serious risk across a wide area. Their use has broadened investigations into corrosion obstruction in various circumstances1-3. Many analysts have focused their efforts on finding more active and safe inhibitors to diminish the corrosive rate and security angles in MS. Among several approaches of preventing corrosion, the inhibitor usage is arguably the utmost effective method to ensuring food safety, especially in acidic environment 4-6. Combinations derived from natural sources used as a consumption inhibitors to prevent metal deferment. It is often related with real adsorption or artificial, including a control change of the adsorbed material and a transfer of charge from one part to another part7–10. The electron influence by giving on the particle, pulling of electron out or bunches which is responsible for adsorption, and the underlying properties of concentrated natural mixtures, the presence of heteroatoms and pi-electrons, cause more prominent inhibitor adsorption on the MS, were all carefully considered 11–13. In line with these claims, this investigation focused on the occurrence and nonappearance of the inhibitor, specifically at different low carbon steel (MS) in 1N Hydrochloric acid..
Materials and Method
Preparation of plate
5 cm x 1 cm size of MS plates were measured and made into piece of which comprise of (in rate) % Carbon-0.016; Si=0.008; Manganese-0.193; Silicon-0.017; Phosphorous-0.008; Nickel-0.012; Molybdenum -0.016; Chromium-0.040 and Iron-99.689% were used for this study. The plates were punched with sequence of numbers and cleaned with various grade of emery sheet and degreased using acetone.
Inhibitor Solution
To carry out de-aeration of the electrolytes, preparation of all solutions were prepared by double aerated distilled water using NICE brand Analytical Reagent chemicals. Solutions of 1N HCl acid were made by twice distilling water. Additionally, solutions of (4-4-ABPFM) at various concentrations (PPM) were made.
Weight loss measurement
A solution of 1N HCl acid was used to dip MS (mild steel) specimens at different inhibitor doses and temperatures. After being removed, the samples were washed in double aerated distilled water and weighed to determine the amount of mass lost. Inhibiton efficiency (IE%) formula was derived from this equation14
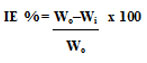
Where, Absence of inhibitor- WO, presence of inhibitor- Wi
Electrochemical Studies
Both Impedance and Polarization studies were completed utilizing at different temperatures utilizing 1N HCl corrosive as the medium. A Pt-platinum terminal and a SCE-immersed calomel cathode were used as aide and reference terminals, separately, and a mild steel example with a 1 cm2 uncovered region filled in as the functioning anode. To decrease the ohmic likely drop, a fine Luggin narrow was utilized to unequivocally situate the reference terminal’s tip very close to the outer layer of the functioning cathode15,16. The electrochemical workstation likewise diminished the leftover uncompensated obstruction. A possible scope of – 800 to – 200 and an output in the pace of 0.01mV s-1were performed for potentiodynamic polarization study. The applied ac bother signal for the electrochemical impedance tests was around 10 mV inside the recurrence scope of 1Hz to 1KHz, and they were led in a similar arrangement as the potentiodynamic polarization examinations. At open circuit voltage, all electrochemical impedance estimations were made. The following equation is utilized to ascertain the level of restraint effectiveness from the upsides of the ongoing thickness (Icorr) [CHI 600D -Electrochemical Workstation]
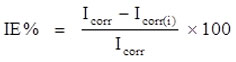
Corrosion current density of inhibitor in absence (Icorr)
Corrosion current density of inhibitor in presence (Icorr(i))
Analogously, an AC impedance study was finished with a repetition range of ten kHz to 0.01 Hz. The certified and imaginary parts of the impedance were shown in Nyquist charts. Separate determinations of the course of action resistance (Rs) and complete check (Rt) blocks were made using the Nyquist plot’s Z’ turn. The qualification between Rt and Rs respects determines the resistance to charge movement. The Cdl views were offered by the condition.
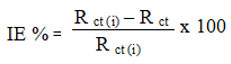
SEM Analysis
Using surface analytical methods at (4-4-ABPFM) inhibitor in both blank and inhibited solution, the metal surface was examined. The mild steel specimen was polished using several types of SiC sheet, then splashed with demineralized water, cleaned with acetone, dried in desiccators, and exposed to three different inhibitor solutions17. A HITACHI-3000 H MODEL SEM-scanning electron microscope was used to record the surface of mild steel.
Result and Discussion
Weight Loss Method
Using (4-4-ABPFM) as a corrosion inhibitor against 1N HCl, weight loss was assessed at two different temperatures. Table 1 presents the findings.
Table 1: Weight Loss measument of (4-4-ABPFM) at two different temperatures.
|
S.No |
Conc. of inhibitor PPM |
Temperature |
|||
|
303K |
313K |
||||
|
CR |
IE |
CR |
IE |
||
|
1. |
Blank |
24.5689 |
— |
85.4759 |
— |
|
2. |
10 |
15.0139 |
38.89 |
60.9184 |
28.73 |
|
3. |
20 |
13.5142 |
44.99 |
56.2342 |
34.21 |
|
4. |
40 |
10.9022 |
55.62 |
54.1285 |
36.67 |
|
5. |
60 |
9.9032 |
59.69 |
51.6987 |
39.51 |
|
6. |
80 |
7.6523 |
68.85 |
45.9872 |
46.19 |
|
7. |
100 |
6.3285 |
74.24 |
41.9587 |
50.91 |
 |
Figure 1: Corrosion Rate of (4-4-ABPFM) in 1N HCl at two different temperatures. |
 |
Figure 2: Inhibition Efficiency of (4-4-ABPFM) in 1N HCl at two different temperatures. |
In accordance with Table 1, at an optimal concentration (100PPM) at all temperatures. The inhibitor shows a better efficacy of 74.24% in comparison to the inhibition efficiency at 303K. After the inhibitor addition at two different temperatures causes the corrosion rate to decrease, as shown in Figure 1, and rises with increasing inhibitor concentration, as indicated by the graph that shows the statistically significant change in inhibition efficiency. By drawing a graph that, respectively, displays the rate of corrosion and the inhibition efficacy in Figure 2. It was found that (4-4-ABPFM) was inhibited at 2 hours with an efficiency of 74.24% in 303K and 50.91% in 313K in 1N HCl.
Potentiodynamic Polarization
Using the inhibitor (4-4-ABPFM), the Ecorr- corrosion potential, Icorr- corrosion potential current , anodic and cathodic Tafel slopes (a & b), and corrosion current (Icorr) were measured and calculated in 1N HCl. The results of the experiment at three distinct temperatures, both with and without the inhibitor, are shown in Figures 3 and 4. The matched Tafel parameters were displayed in Table 2.
Table 2: Potentiodynamic Polarization Studies of 1 N hydrochloric acid in the absence and presence of the (4-4-ABPFM) at various temperatures.
|
Temperature |
Conc.(PPM) |
-βa (mV dec-1) |
-βc (mV dec-1) |
Ecorr (mV) |
icorr (mA cm-2) |
Corrosion rate (mmpy) |
IE % |
|
303K |
Blank |
23.359 |
7.709 |
-576 |
29.852 |
196.52 |
— |
|
10 |
16.156 |
6.902 |
-572 |
23.160 |
173.20 |
22.41 |
|
|
20 |
6.088 |
4.809 |
-544 |
21.844 |
152.54 |
26.82 |
|
|
40 |
9.062 |
5.182 |
-521 |
19.510 |
112.56 |
34.64 |
|
|
60 |
5.470 |
4.241 |
-483 |
15.753 |
98.75 |
47.22 |
|
|
80 |
7.525 |
4.496 |
-428 |
10.789 |
88.79 |
63.85 |
|
|
100 |
16.753 |
5.593 |
-411 |
7.825 |
72.68 |
73.78 |
|
|
313 K |
Blank |
5.326 |
4.917 |
-542 |
18.646 |
216.67 |
— |
|
10 |
15.685 |
7.595 |
-507 |
15.723 |
205.73 |
15.67 |
|
|
20 |
10.632 |
5.026 |
-494 |
10.825 |
188.95 |
37.94 |
|
|
40 |
11.756 |
5.205 |
-490 |
9.875 |
174.52 |
47.03 |
|
|
60 |
14.795 |
6.048 |
-482 |
9.230 |
156.23 |
48.49 |
|
|
80 |
11.433 |
3.858 |
-463 |
8.251 |
142.35 |
50.74 |
|
|
100 |
15.755 |
4.097 |
-431 |
7.754 |
130.61 |
52.41 |
 |
Figure 3: Tafel curves on Mild Steel in 1 N HCl using (4-ABPFM)–303K. |
 |
Figure 4: Tafel curves on Mild Steel in 1 N HCl using (4-ABPFM)- 313K. |
The values of Ecorr are slightly more positive in the presence of an inhibitor, as shown by Table 2 data and plot. This suggests that the inhibitor (4-4-ABPFM) is preventing mild steel from corroding in 1N HCl acid solution by controlling cathodic processes that block the metal’s active sites. The fact that an inhibitor strongly polarizes the cathode suggests that it is a cathodic inhibitor. Tafel slope results at three different temperatures demonstrate the inhibitor’s anti-corrosive qualities, which are caused by the cathode’s corrosion processes. The lowered value of Icorr indicates the efficiency of the inhibitor and its interaction with a metal surface at three distinct temperatures. The highest percentage of inhibition (73.78%). 303K > 313K is the inhibitory efficacy order.
AC Impedance Method
As for open circuit potential, Figures 5 and 6 depict an impedance diagram for MS in 1N hydrochloric acid with and without (4-4-ABPFM), using a stable frequency range of 10 kilo Hertz to 0.01 Hertz. The calculated impedance values are listed in Table 3.
Table 3: Mild Steel in 1N HCl medium with (4-4-ABPFM) at two different temperatures- AC impedance study.
|
Temperature |
Concentration
|
Rct (Ohm cm2) |
Cdl (µF/ cm2) |
I.E % |
|
303K |
Blank |
21.98 |
127.99 |
— |
|
10 |
28.32 |
124.56 |
20.40 |
|
|
20 |
30.01 |
102.89 |
24.89 |
|
|
40 |
38.23 |
81.56 |
41.04 |
|
|
60 |
57.12 |
78.51 |
60.53 |
|
|
80 |
63.25 |
69.69 |
64.36 |
|
|
100 |
85.87 |
45.68 |
73.75 |
|
|
313K |
Blank |
22.17 |
1344.40 |
— |
|
10 |
26.36 |
349.85 |
15.89 |
|
|
20 |
38.49 |
276.80 |
23.40 |
|
|
40 |
42.20 |
259.20 |
37.46 |
|
|
60 |
45.07 |
225.78 |
42.80 |
|
|
80 |
50.12 |
223.00 |
45.76 |
|
|
100 |
53.23 |
163.60 |
51.35 |
 |
Figure 5: AC Impedance curves of (4-4-ABPFM) for MS in 1 N Hydrochloric acid at 303K. |
 |
Figure 6: AC Impedance curves of (4-4-ABPFM) for MS in 1 N Hydrochloric acid at 313K. |
The inhibitor is added, and the table and Nyquist plots demonstrate that this causes the Rct value to rise and the Cdl value to decrease. The results demonstrate that a large charge transfer resistance is linked to a slow corroding mechanism. The boosting characteristics of the inhibitor cause the metal’s capacitance to decrease. Due to local dielectric constant value decreases because there is a slight drop in the results of Double layer capacitance. Formation of electrical double-layer’s breadth verified (4-4-ABPFM) was dynamically adsorbed at the metal contact. At three different temperatures, the inhibitor in 1N HCl takes on a semicircular form, suggesting that the surface of MS and inhibitor are transferring charges in a controlled way. The inhibitor’s optimum concentration of 100 ppm of (4-4-ABPFM) at 303 K was found to have an inhibitory effectiveness of 73.75% in 1N HCl. The comparison of the mass loss measurement, polarization experiments, and AC impedance testing findings demonstrates the inhibitor’s efficacy.
SEM Analysis
Surface morphological examinations clearly show the inhibitor’s effect, as does the CB-corrosion behavior of low carbon steel (MS) in the nonexistence and existence of (4-4-ABPFM), as demonstrated by SEM study. The specimen that was used in the SEM experiment was immersed in 1N HCl acid at ideal concentrations, without and with an inhibitor, at three distinct temperatures. The specimens were taken out of the inspection, cleaned, and promptly rinsed with rectified spirit before being dried and conserved. A somewhat large oxide inclusion has formed over the metal surface.
 |
Figure 7: Surface morphological image of MS in 1N HCl Blank medium |
 |
Figure 8: SEM image of MS in 1NHCl Medium with the presence (4-4-ABPFM) (a)303K (b) 313K |
Figure 7 shows a SEM picture of an MS that was immersed in 1N HCl for two hours without the aid of an inhibitor, demonstrating the significant metal breakdown brought on by the aggressively acidic environments. Figure 8(a and b) displays the surface analysis pictures of MS immersed in 1HCl at (4-4-ABPFM) at three distinct temperatures. A strong protective layer is formed on the metal surface as a result of the inhibitor’s molecules interacting with one another. This is clear evidence of the decreasing rate of surface coverage.
Conclusion
The following are the results of the present study on the application of (4-4-ABPFM) in 1N HCl acid solution to regulate mild steel corrosion at two distinct temperatures. The inhibiton efficiency of (4-4-ABPFM) is higher in an acidic medium. The efficiency in (4-4-ABPFM) was measured in 1N HCl at 303K. By using the (4-4-ABPFM) inhibitor, the chemical under investigation in 1N HCl inhibits both the cathodic and anodic shift, functioning as a mixed type inhibitor, according to polarization measurements. Impendence experiments reveal that when electron-donating groups are present on the inhibitor, the double layer capacitance reduces and the charge transfer resistance rises.. It has been shown that a major influence on the inhibition mechanism is the kind of functional atoms and substitutents group of the inhibitor. Likewise, SEM demonstrates the inhibitor’s increased efficacy.
Acknowledgement
The author acknowledges Dr.N.Bhadusha for his guidance and also for Dr.Ganavel for his support to carry out the research work.
Reference
- Ning S.G.; Shi M. L.; J Chin Soc. Corros, Pro.,1990, 383.
- Mehdi B. EI.; Mernari B.; Traisnel M.; Bentiss F.; Lagrenee M.; MaterChem Phys., 2002, 77, 489.
CrossRef - Govindaraju K. M.; Gopi. D.; Kavitha L.; J Appl Electrochem., 2009,39,269
CrossRef - Anand B.; Balasubramanian V.; E-Journal of Chemistry., 2011, 8(1),226
CrossRef - Zerga B.; Sfaria M.; Rasis Z.; Ebn Touhami M.; Taleb M.; Hammouti B.; Imelouane B.; Elbachiri A.; Mater Tech.,2009, 97,297.
CrossRef - Chaieb E.; Bouyanzer A.; Hammouti B.; Benkadour M.; Appl Surf Sci., 2005,246,199
CrossRef - Bouklah M.; Hammouti B.; Port Electrochim Acta., 2006, 24,457
CrossRef - Bouyanzer A.; Majidi L.; Hammouti B.; Phys Chem News., 2007,37,70.
- Matheswaran P.; E-Journal of Chemistry., 2011,7(3),1090
CrossRef - Ferreira E S.; Giacomelli C.; Gicomelli F.C.; Spinelli A.; Mater Chem Phys., 2004,83,129
CrossRef - Bouklah M.; Ouassini A.; Hammouti B.; Idrissi A.E.I.; Appl Surf Sci., 2006,252,2178
CrossRef - Anand B.; Jayandran M.; Balasubramanian V.; Asian Journal of Chemistry., 2011,23(5) 2106.
- Govindaraju K. M.; Gopi D.; Kavitha L.; J Appl Electrochem., 2009,10,263
- Abiola O. K.; Oforka N. C.; Ebenso E. E.; Bulletin of Electrochemistry., 2004, 20,409
- Bentiss F.; Lebrini M.; Lagrene M.; Corros Sci., 2005,47,2915.
CrossRef - Singh A.; Singh V.K.; Quraishi M. A.; Int. J. Corros.,2010,10,275
CrossRef - Abd El-Rehim S. S.; Rwfaey S. A.; Taha F.; Saleh M..B.; Ahmed R. A.; J. Appl. Electrochem.,2001,31,429
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










