Effect of NaCl and KCl on the Micellization of Sodium Dodecyl Sulfate and Sodium Dodecyl Benzene Sulfonate in Presence of Cephradine Monohydrate Through Conductivity
Department of Chemistry, Chittagong University of Engineering and Technology, Raozan, Chattogram, Bangladesh.
Corresponding Author E-mail: roksana@cuet.ac.bd
DOI : http://dx.doi.org/10.13005/ojc/400323
Article Received on : 20 Mar 2024
Article Accepted on : 17 Jun 2024
Article Published : 21 Jun 2024
Reviewed by: Dr. Ajmer Singh
Second Review by: Dr. Neelaveni Thangavel
Final Approval by: Dr. Ioana Stanciu
The effect of NaCl and KCl on the micellization of sodium dodecyl sulfate (SDS) and sodium dodecyl benzene sulfonate (SDBS) surfactants in cephradine monohydrate (CPDM) has been studied by conductance measurement. In this study, we have examined the critical micelle concentration (CMC) of surfactant solutions in drug-salt systems by conductance measurement in an aqueous medium and electrolyte solutions. In this study, CMC were calculated through piece-wise linear model by identifying a distinct and abrupt change in the specific conductivity (G) as the surfactant concentration increased. In all the experimental cases examined, a single CMC was identified for both the CPDM + SDS and CPDM + SDBS systems. The presence of CPDM in an aqueous solution decreases the critical micelle concentration (CMC) of surfactants, thereby increasing the degree of micellization. The CMC values were found to favorable with an increase of salt NaCl and KCl concentrations. And very tremendously different results were found between CPDM + SDS/SDBS and CPDM + SDS/SDBS+KCl and CPDM + SDS/SDBS+NaCl systems. The result shows very remarkable information about the micellization of surfactants in the presence of drug and salt solutions
KEYWORDS:CMC; Micelle; Micelle ionization Value; Specific Conductivity
Download this article as:| Copy the following to cite this article: Islam M. S, Khatun R, Gupta A. D. Effect of NaCl and KCl on the Micellization of Sodium Dodecyl Sulfate and Sodium Dodecyl Benzene Sulfonate in Presence of Cephradine Monohydrate Through Conductivity. Orient J Chem 2024;40(3). |
| Copy the following to cite this URL: Islam M. S, Khatun R, Gupta A. D. Effect of NaCl and KCl on the Micellization of Sodium Dodecyl Sulfate and Sodium Dodecyl Benzene Sulfonate in Presence of Cephradine Monohydrate Through Conductivity. Orient J Chem 2024;40(3). Available from: https://bit.ly/3S5f94b |
Introduction
Compounds known as surfactants are made up of lyophilic groups that have a high affinity to the solvent and lyophobic groups that have a very weak one. Such types of structures are called amphiphatic compounds1,2. Surfactants have different uses in textiles, cosmetic and domestic products, metal extraction, food processing, nanotechnologies, oil recovery, and pharmaceuticals for hydrophobic drug solubilization in aqueous phase, as parts of emulsions, plasticizers in semisolid delivery systems, surfactant micelle vehicles for transdermal and oral drug delivery, and as agents to improve drug absorption and penetration3,4.The surfactant molecules form very well ordered micelles, bi-layers, hexagonal, monolayers, or cubic phases that aggregate themselves5–7. These different phases are affected by the structure of surfactants, the space gap between the hydrophilic and hydrophobic parts and the extent of hydrophilic-hydrophobic balance. The process of inter-conversion occurs depending on factors such as the pH of the medium, temperature, and ionic strength7–9. The critical micelle concentration (CMC) represents the concentration level at which micellar structures begin to form7,10. In the micelle, the micelle core part contains the non-polar moieties portion and outside part contains the polar part of the surfactant molecule7. Then they are arranged by solvent as a spherical arrangement for both the anionic and cataionic surfactant micelles at CMC11,12. The ionic surfactants improve drug permeability through the skin13.
The surfactants can be used in their micellized form for feebly soluble drugs to increase the dissolution rate13. The membrane function is affected by surfactants and the absorption, and penetration of drugs across the gastrointestinal wall are improved by the surfactants13,14. Surfactants have a better performance in terms of absorption and release of drugs in the blood stream in micellar form 14–19. Because of the similarity between the surfactant micelle and the biological membrane, this concept of micelle formation could prove beneficial for studying the different modes in which the chosen drugs bind20. As the micelles have a very stable structure and have an appropriate size, they can congregate easily in the targeted areas21.
 |
Scheme 1: Structure of SDS, SDBS and CPDM. |
Cephradine is a first-generation cephalosporin and beta-lactam antibiotic22. It effectively combats both gram-positive and gram-negative bacteria. Its application spans across treating upper respiratory tract infections, lower respiratory tract infections, urinary tract infections, as well as skin and soft tissue infections23. SDS is an anionic surfactant24. It is used in our everyday items, such as foods, pharmaceutical formulations, cleaning agents, and research purposes also25–27. SDBS is also an anionic surfactant. It is well known as a surface active compound. It is used in chemical, biochemical, and industrial work. It shows antifungal properties28,29. As NaCl and KCl is naturally found in the human body and sometimes NaCl is pushed along with glucose solutions, so they may affect the physicochemical interaction of membranes with drugs7,30. A number of studies on the interaction with drug molecules have been reported in the literature16,21 but for our best knowledge, it is necessary to explore the interactions of cephradine on the micellization of SDS and SDBS in the presence of NaCl and KCl31.
Materials and Method
Materials
Table 1: Specifications of Chemicals
|
Chemical name |
Molar mass/kg.mol-1 |
Purity declared by supplier |
origin |
|
Cephradine monohydrate(CPDM) |
0.34941 |
0.995 |
SKF pharmaceuticals Ltd |
|
Sodium dodecyl sulfate(SDS |
0.28838 |
0.990 |
Merck(Germany) |
|
Sodium Chloride(NaCl) |
0.05844 |
0.995 |
Merck in Germany |
|
Potasium Chloride(KCl) |
0.07455 |
0.995 |
Merck in Germany |
All the materials utilized in this experiment were analytical reagent grade and were used without any purification. The surfactants, SDS and SDBS were collected from Merck(Germany).The employed drug Cephradine (Micronised) was collected from SKF Pharmaceuticals Ltd. in Bangladesh. The sodium chloride (NaCl) was acquired from a research lab in India, while potassium chloride (KCl) was procured from Merck in Germany. All solutions were made using deionized water with a specific conductivity lower than 2 µScm-1.
Method
The specific conductivity of CPDM+H2O+SDS, CPDM +H2O+SDS+NaCl/KCl, CPDM +H2O+SDBS, CPDM +H2O+SDBS+NaCl/KCl systems were measured through Hanna EC/TDS/Salinity Benchtop Meter – HI2550 having a cell constant 1cm-1 and an accuracy of ±.05 µScm-1.The FA2204 analytical balance was used for weight measurements. All the experiments were performed at 25°C, which was controlled by the shaking water bath model SHWB-30. The experiment was conducted according to the procedure outlined in the literature29,32,33.
The stock solutions of SDS(0.15mol/kg) and SDBS(0.15 mol/kg) were prepared by solvent-1,2 and 3 (solvent-1(CPDM + H2O), solvent-2(CPDM + H2O+NaCl), solvent-3(CPDM + H2O+KCl)), and then the SDS and SDBS solutions were transferred gradually into a beaker containing the corresponding 80g solvent-1,2 and 3 for targeting the desired concentration. After adding the SDS/ SDBS solution every time it was mixed properly. A conductivity meter was used to measure the values of G and each time a stock solution of surfactants was added to the solvent. Plotting of the G values versus the corresponding surfactant concentrations has been done.
Results and discussion
Conductometric study is one of the basic structurally susceptible methods for analysis aggregation or micellar systems and this method is widely applied to evaluate critical micelle concentrations (CMCs). As the concentration of an ionic surfactant rises within a solution, a change is noticeable in the conductivity data curve near the critical micelle concentration (CMC). Beyond this point, the increase in conductivity becomes less pronounced, indicating a slower rate of change. This occurrence is attributed to the reduced mobility of micelles in an electric field compared to unassociated surface-active ions due to their larger size. Some graphs of the specific conductivity of SDS and SDBS in aqueous, CPDM +H2O, CPDM +H2O+NaCl, and CPDM +H2O+KCl solutions are graphically represented in Figures-1, 3 and the second derivatives are presented graphically in Figures-2, 4
To determine the critical micelle concentration (CMC) precisely, the conductance value as a function of molality was fitted to the following piece-wise linear model;
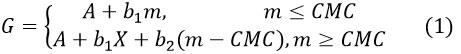
Where A indicates the intercepts b1 and b2 refer to the experimental slopes in the before and after-CMC regions, respectively. Table- 2 contains the values of the fitting parameters accompanied by their respective standard uncertainties, and CMC’s are graphically shown in Figures-5, 6 against the concentrations of NaCl and KCl. The conductivity values are calculated using eq (4.1). The second derivative data follows the Gaussian peak function, which indicates the existence of a breaking point in conductivity versus molality data. The degree of dissociation of micelle and the degree of association of micelle α= (1-β), were calculated in the pre- and post-CMC regions. The values of α and β offer significant physicochemical insights into the properties of the surfactant micelle34.
The conductivity of all systems was observed to progressively rise as the concentration of surfactant increased (Figure-1, 3). Because ionic surfactants like SDS and SDBS get dissociated and produce surfactant ions and counter ions. These ions are responsible for conductivity. As the concentration increases, the number of ions increases, resulting in higher conductivity. But after a definite concentration, surfactant ions get self-assembled and then conductivity increases at a slower rate. In this specific concentration, the plot of surfactant concentration vs. conductivity shows a breakpoint at CMC. In all our experiments we have obtained only one CMC.
CPDM + SDS and CPDM + SDBS have CMC values that are less than those of pure SDS and SDBS in water, indicating that the addition of CPDM promotes the formation of surfactant micelles. For both SDS and SDBS, the CMC values dropped as NaCl and KCl concentrations increased (Figures-5, 6). Thus, the CPDM supported surfactant micelles are stabilized by the addition of NaCl and KCl. The term “salting effect” refers to the phenomenon wherein CMC values decrease when neutral salts such as NaCl and KCl are added7,16. Other research teams have similarly noted a decrease in the CMC when electrolytic salts are introduced during the micellization process involving interactions between ionic surfactants and drug surfactants35–37. Both NaCl and KCl are potent electrolytes. By complete dissociation, they release ions and water structure become stable by solvating ions, this leads to a significant salting-out effect on the hydrophobic portion of the surfactant monomer. The existence of NaCl and KCl aids in neutralizing surface charges, thereby reducing repulsion between head groups and promoting micelle formation5,7. In comparison to NaCl and KCl, CMC decreases more when KCl is present and less when NaCl is present for both SDS and SDBS [Figure -9, 10]. The van der Waals radius (R) and valence (Z) ratio can be used to describe it. Larger Z/R cations are more hydrated and contribute more water structure36. Because these ions can salt out the hydrophobic groups of the surfactants in the aqueous phase, they have a greater effect on the CMC value. In the presence of KCl, there is a greater drop in CMC because the Z/R value for K+ ions is higher38.
From the slope before (Sb) and after (Sa) CMC, α values can be obtained using the equation, α= Sa/Sb, and β values can be computed from the relation β= 1-α. Important physiochemical features of the surfactant micelle are provided by the values of α and β. In our current study we have a good agreement with references16. The lower alpha values observed for CPDM +SDS indicate that it exhibits weaker interaction with SDS micelles, resulting in less efficient solubilization compared to SDBS micelles32.
 |
Figure 1: Conductivities of (a). SDS+water+CPDM (0.001mol/kg), (b). SDS+water+ NaCl( 0.001mol/kg)+CPDM (0.001mol/kg), |
 |
Figure 2: Conductivities of a). SDBS + water , b). SDBS + water + CPDM (0.001mol/kg), c). SDS + water + NaCl (0.001mol/kg)+CPDM (0.001mol/kg). |
 |
Figure 3: Conductivities of (a). SDBS + water + CPDM (0.001mol/kg), (b). SDBS + water + NaCl (0.001mol/kg) + CPDM (0.001mol/kg) |
 |
Figure 4: Conductivities of e). SDBS + water , f). SDBS + water + CPDM (0.001mol/kg). |
 |
Figure 5: CMC of SDS in Cephradine (.001mol/kg)+NaCl/KCl systems(( – Represents CMC in presence of NaCl and ◯ – represents CMC in presence of KCl). |
 |
Figure 6: CMC of SDBS in Cephradine (0.001mol/kg) +NaCl/KCl systems ( – Represents CMC in presence of NaCl and ◯ – represents CMC in presence of KCl). |
Table 2: Critical micelle concentration, CMC of SDS and SDBS in water and in Water+ NaCl/KCl+CDPM solutions.
|
Systems |
CMC(X)(m/ mol·kg−1) |
α |
β |
|||
|
SDS+Water |
0.00819 |
0.67011 |
0.32989 |
|||
|
SDBS+Water |
0.00337 |
0.91377 |
0.08623 |
|||
|
m/ (mol·kg−1) of NaCl |
CMC of SDS in |
CMC of SDS in |
α |
β |
||
|
SDS+ |
SDS+ |
SDS+ |
SDS+ |
|||
|
0.000 |
0.00814 |
0.00814 |
0.64770 |
0.64770 |
0.3523 |
0.3523 |
|
0.001 |
0.00735 |
0.00718 |
0.61409 |
0.63011 |
0.38591 |
0.36988 |
|
0.003 |
0.00678 |
0.00540 |
0.55409 |
0.63701 |
0.44591 |
0.36701 |
|
0.005 |
0.00509 |
0.00468 |
0.51438 |
0.76511 |
0.48562 |
0.23849 |
|
0.007 |
0.00481 |
0.00438 |
0.56097 |
0.63360 |
0.43903 |
0.3664 |
|
0.009 |
0.00440 |
0.00352 |
0.55209 |
0.37341 |
0.44791 |
0.62659 |
|
m/ (mol·kg−1) of KCl |
CMC of SDBS in |
CMC of SDBS in |
SDBS+ |
SDBS+ |
SDBS+ |
SDBS+ |
|
0.000 |
0.00282 |
0.00282 |
0.88359 |
0.88359 |
0.11641 |
0.11641 |
|
0.001 |
0.00237 |
0.00232 |
0.91648 |
0.90677 |
0.08352 |
0.09323 |
|
0.003 |
0.00209 |
0.00161 |
0.94927 |
0.90369 |
0.05073 |
0.09631 |
|
0.005 |
0.00170 |
0.00154 |
0.93559 |
0.91173 |
0.06441 |
0.08827 |
|
0.007 |
0.00140 |
0.00129 |
0.89436 |
0.90959 |
0.10564 |
0.09041 |
|
0.009 |
0.00129 |
0.00103 |
0.77913 |
0.86314 |
0.22087 |
0.13686 |
Conclusion
A comprehensive conductometric study was conducted to investigate the interaction between the antibiotic CPDM and the anionic surfactants SDS and SDBS, both in aqueous solutions and in salt solutions. A significant interaction between the drug and surfactants was observed in the presence of the salts NaCl and KCl when each was added independently. Our results indicate that the interaction between the drug and SDS, SDBS is due to strong hydrophobic interactions. The CMC values of the surfactants were consistently lower than the ideal CMC values, with these values further decreasing in the presence of NaCl and KCl. The reduction in CMC as the concentration of KCl or NaCl rises is ascribed to the diminished electrostatic repulsion between the charged head groups of surfactants in the presence of CDM, thereby promoting the creation of surfactant micelles. In comparison to NaCl, there was a greater decrease in CMCs when KCl was present. We can learn more about the nature of the drug-surfactant interaction thanks to the data. It can be extremely important for both medication delivery systems and improved drug formulation.
Acknowledgement
The Chittagong University of Engineering & Technology, Bangladesh’s Department of Chemistry is acknowledged by the authors for providing the funding and lab space needed to complete this research project.
Conflict of Interest
There is no conflict of interest.
References
- Suhail, M.;Kumar Janakiraman, A.; Khan, A.; Naeem, A.; Faisal Badshah, S.,J Pharm Pharm. 2019;(October):72-82. doi:10.15436/2377-1313.19.2601
- Rebello, S.; Asok,A.K.; Mundayoor, S.; Jisha,M.S.,Surfactants : Chemistry , Toxicity and Remediation.; 2013. doi:10.1007/978-3-319-02387-8
CrossRef - Kumar, D.; Rub, M.A., J Mol Liq. 2019;274(Ii):639-645. doi:10.1016/j.molliq.2018.11.035
CrossRef - Sekhon, B.S., J Pharm Technol Res Manag. 2013;1(1):43-68. doi:10.15415/jptrm.2013.11004
CrossRef - Rahman, M.; Hoque, M.A.; Rub, M.A.; Khan, M.A., Chinese J Chem Eng. 2019;27(8):1895-1903. doi:10.1016/j.cjche.2018.10.022
CrossRef - Kumar, D.; Rub, M.A.; Asiri, A.M., R Soc Open Sci. 2020;7(7). doi:10.1098/rsos.200775
CrossRef - Hoque, M.A.; Rahman, M.M.; Alam, M.M., J Mol Liq. 2021;326:115337. doi:10.1016/j.molliq.2021.115337
CrossRef - Sharma,R.; Mahajan, R.K., RSC Advances. 2012, 2, 9571–9583. doi:10.1039/c2ra21020g
CrossRef - Wang, D.; Kowalczyk ,B.; Grzybowski ,B.A., Langmuir. 2010;26(23):13770-13772. doi:10.1021/la102635w
CrossRef - Khatun,M.R.,;Islam M.M.; Rima,F.R.; Islam,M.N., J Chem Eng Data. 2016;61(1):102-113. doi:10.1021/acs.jced.5b00317
CrossRef - Qashqoosh, M.T.A.; Alahdal, F.A.M.; Manea, Y.K.; Zakariya, S.M.; Naqvi, S., Chem Phys. 2019;527(February):110462. doi:10.1016/j.chemphys.2019.110462
CrossRef - Wang,R.; Tang,Y.,;Wang, Y., Langmuir.2014, 30, 1957−1968. dx.doi.org/10.1021/la500025k
CrossRef - Bhardwa.j. V.; Bhardwaj, T.; Sharma, K., RSC Advances,Vol 4.; 2014. doi:10.1039/c4ra02177k
CrossRef - Rosen,M.J.,Surfactants and interfacial phenomena(Third edition).2012 . doi:10.1016/0166-6622(89)80030-7
CrossRef - Kumar, D.; Hidayathulla, S.; Rub, M.A., J Mol Liq. 2018;271:254-264. doi:10.1016/j.molliq.2018.08.147
CrossRef - Kumar, D.; Rub, M.A., J Phys Org Chem. 2019;32(11):1-9. doi:10.1002/poc.3997
CrossRef - Kumar, D.; Rub, M.A.; Azum, N.; Asiri, A.M., J Phys Org Chem. 2018;31(1):1-12.
CrossRef - Patra, N.; Mal, A.; Dey, A., J Mol Liq. 2019;280:307-313. doi:10.1016/j.molliq.2019.02.002
CrossRef - Kumar, D.; Azum, N.; Rub, M.A.; Asiri, A.M., J Mol Liq. 2018;262:86-96. doi:10.1016/j.molliq.2018.04.053
CrossRef - Pathania,L. ;Chauhan,S., Journal ofMolecular Liquids.2019.doi.org/10.1016/ j.molliq.2019.112210
CrossRef - Elektrolit,.K.;Setil, M.; Bromida,T., Sains Malaysiana..2017; 46(5):733–741 dx.doi.org/10.17576/jsm-2017-4605-08
CrossRef - Awaz,M.N.; Rayne,M.S.; Sultana, N.S., Acta Chromatographica. 2011;23:205-213. doi:10.1556/AChrom.23.2011.2.2
CrossRef - Mehta,D.; Sharma A.K.; Inventi Rapid: Molecular Pharmacology.2016;2016(1):1-6.
- Khatun,M.R.; Islam,M.M.; Islam,M.D.N.; Rhaman,M.D.M.;Nath, R.K., Asian J Chem. 2019;31(5):1113-1127. doi:10.14233/ajchem.2019.21844
CrossRef - Bhattarai, A., J Mol Liq. 2019;292. doi:10.1016/j.molliq.2019.111352
CrossRef - Li, Y.; Song, J.; Tian. N., Int J Pharm. 2014;473(1-2):316-325. doi:10.1016/j.ijpharm.2014.07.011
CrossRef - Alana,D.; Azevedo, A. De.; Peixoto,R.M., Arq. Inst. Biol. 2019 .v.86, 1-9, doi:10.1590/1808-1657000752018
CrossRef - Yefei,W.; Huaimin,X.; Weizhao,Y.;Baojun,B.; Xinwang, S., Pet.Sci.2011.8:463-476 doi:10.1007/s12182-011-0164-7
CrossRef - Patino,A.Aj.; Dias ,F.GM.; Ferreira,G.M.D.; Coelho,Y.L.; Hudson,E.A.;Dos,S.P., Silva, L.H.M., Colloids and Surfaces A: Physicochemical and Engineering Aspects .2020. doi: https://doi.org/10.1016/j.colsurfa.2020.12443
- Khatun,M.R.; Islam,M.N.; Orient J Chem. 2012;28(1):165-187. doi:10.13005/ojc/280123
CrossRef - Khatun,M.R.; Islam M.M,;Islam M.D.; Orient J Chem. 2020;36(05):863-870. doi:10.13005/ojc/360510
CrossRef - Aktar, S.; Saha, M.; Mahbub, S., J Mol Liq. 2020;306. doi:10.1016/j.molliq.2020.112880
CrossRef - Elarbi,F.M.; Janger,A.A.; Abu-sen,L.M.; Ettarhouni,Z.O., American Journal of Engineering Research.2020.8:118-126.
- Grueso,E.; Cerrillos,C.; Hidalgo,J., Langmuir.2012.28, 10968−10979 dx.doi.org/10.1021/la302373m
CrossRef - Sood, A.K.; Aggarwal, M., J Chem Sci. 2018;130(4):1-7. doi:10.1007/s12039-018-1446-z
CrossRef - Akram, M.; Bhat. I.A., Kabir-ud-Din, Colloids Surfaces A Physicochem Eng Asp. 2016;493:32-40. doi:10.1016/j.colsurfa.2016.01.005
CrossRef - Zdziennicka,A.; Szymczyk,K.; Krawczyk,J.; Janczuk,B., Fluid Phase Equilibria. 2012.322– 323:126-134. doi:10.1016/j.fluid.2012.03.018
CrossRef - Ren, Z.H., Ind. Eng. Chem. Res. 2015, 54, 9683−9688 doi:10.1021/acs.iecr.5b02169
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










