Synthesis of Some Noval Qunazolinone Derivatives for their Anticonvulsant Activity
Phool Singh Yaduwanshi1* , Sheelu Singh1
, Sheelu Singh1 , Prinsi Sahapuriya1
, Prinsi Sahapuriya1 , Priyanka Dubey1
, Priyanka Dubey1 , Jyoti Thakur1
, Jyoti Thakur1 and Savita Yadav2
and Savita Yadav2
1IES Institute of Pharmacy, IES University, Bhopal, (M.P.), Pin 462044, India.
2IES Institute of Technology and Management, IES University, Bhopal, (M.P.), Pin 462044, India.
Corresponding Author E-mail: phoolsinghyaduwanshi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400207
Article Received on : 08 Dec 2023
Article Accepted on :
Article Published : 11 Mar 2024
Reviewed by: Dr. Paras Nath YADAV
Second Review by: Dr. Rebaz Anwar Omer
Final Approval by: Dr. Tanay Pramanik
The quinoline family comprises an appealing group of heterocyclic compounds, with quinazolinones and their synthetic analogs being of particular interest. To synthesize 3- amino 2-phenyl quinazolinones, anthranilic acid and its substituted derivatives were employed as initial materials. The MES method was utilized to evaluate the anticonvulsant activity of the developed substances on albino mice, with phenytoin serving as a benchmark anticonvulsant medication.The synthesized compounds demonstrated noteworthy anticonvulsant activity, comparable to that of established prescription medications. Among these compounds, Compound A-1 exhibited the highest level of activity. This indicates the potential of these synthetic analogs as effective anticonvulsants, with Compound A-1 standing out as particularly promising in this regard.Preliminary results indicate that certain quinazolinone derivatives exhibited promising anticonvulsant effects in the MES test. Further investigation into the mechanism of action and safety profile of these compounds is underway. The structure-activity relationships deduced from this study may guide the design of future anticonvulsant agents based on the quinazolinone scaffold.This research contributes to the ongoing efforts to discover new therapeutic options for epilepsy and provides valuable insights into the potential of quinazolinone derivatives as anticonvulsant agents. The findings underscore the importance of exploring diverse chemical structures in the quest for improved treatments for neurological disorders.
KEYWORDS:An anticonvulsant; Maximal Electroshock (MES); Quinazolinone; Synthesis
Download this article as:| Copy the following to cite this article: Yaduwanshi P. S, Singh S, Sahapuriya S, Dubey P, Thakur J, Yadav S. Synthesis of Some Noval Qunazolinone Derivatives for their Anticonvulsant Activity. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Yaduwanshi P. S, Singh S, Sahapuriya S, Dubey P, Thakur J, Yadav S. Synthesis of Some Noval Qunazolinone Derivatives for their Anticonvulsant Activity. Orient J Chem 2024;40(2). Available from: https://bit.ly/3TcQgmx |
Introduction
Quinazolinone derivatives have emerged as a class of compounds with versatile biological activities, drawing considerable interest in medicinal chemistry. The broad spectrum of biological properties associated with quinazolinones, coupled with their structural diversity, has positioned them as promising candidates for drug development1-3. In particular, quinazolinone derivatives have exhibited a myriad of pharmacological effects, including anticancer4, antimicrobial5, antifungal6, antiviral7, antitumer8, antimalarial9, muscle relaxant10, anti-inflammatory11, anti-tubercular12, and anticonvulsant13 activities, among others. The focus of this study is the synthesis of novel quinazolinone derivatives designed with the specific aim of evaluating their potential anticonvulsant activity. Epilepsy, a neurological disorder characterized by recurrent seizures, remains a significant global health concern. Despite advancements in treatment options, there is a continuous need for the discovery of new and more effective anticonvulsant agents14-17. This research contributes to the ongoing efforts in medicinal chemistry to explore diverse chemical structures for their therapeutic potential. By synthesizing and evaluating novel quinazolinone derivatives, we aim to expand the understanding of their anticonvulsant properties, paving the way for the development of new drugs to address the challenges associated with epilepsy. The investigation involves not only the synthesis of these compounds but also their comprehensive characterization and evaluation using established preclinical models, such as the Maximal Electroshock (MES) method. The outcomes of this research hold the promise of advancing our knowledge in the pursuit of innovative treatments for neurological disorders, with a particular focus on anticonvulsant interventions.
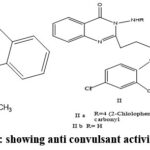 |
Figure 1: showing anti convulsant activity. |
Method and Scheme
Quinazolines were studied using the synthetic method. 18
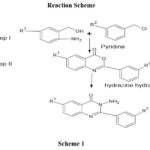 |
Scheme 1 |
Step-I Synthesis
Anthranillic acid or substituted anthranillic acid (0.1M) was dissolved in 60 ml pyridine and then added benzoyl chloride (0.05M) drop wised and stirring the product half an hour and neutralize the product with NaHCo3,recrystallized with ethanol. ‘Tempo’ melting point equipment was used to determine the melting point, and the results were uncorrected. There were also melting points.1440C, 1350C, 1410C, 1780C, 980C, 194-1980C, 184-1880C, 1470C, 1180C, 1780C, 1440C and 1180C and Percentage yield were 78, 64.5, 56, 43, 45, 54, 40, 64.6, 68.4, 64.9, 43, 45, and 38 . IR of KBr (in cm-1): 3076 (C-H, ArH Str), 1758 (C=O Str),1681Cycle C = 0 Str, 1616 (C=N Str), 1512 (C=C Str),1456, (C-N Starching) 839,1HNMR(ppm) CDCl3, 4.531 (s, 3H, -SCH3); 7.483-8.091 (m, 8H, Ar-H). 3.78 (Methoxy proton ),1.7 (Methylene Proton ).4.07(3H, CH3),11.32(s1H,NH).
Physical characteristics of synthesized compounds (I)
Table 1
|
S.No. |
R
|
R’
|
CODE |
Yield (%) |
Color |
m.p. (0C) |
Rf |
||
|
Substituent |
Quantity |
Substituent |
Quantity |
||||||
|
1 |
H |
13.7g |
H |
7.03g |
1a |
78 |
Pale yellow |
124 |
0.8 |
|
2 |
H |
13.7g |
Cl |
8.70g |
1b |
62.5 |
Pale yellow |
135 |
0.12 |
|
3 |
H |
13.7g |
NO2 |
9.20g |
1c |
56 |
Pale yellow |
141 |
0.51 |
|
4 |
H |
13.7g |
OCH3 |
8.55g |
1d |
42 |
Ash grey |
178 |
0.38
|
|
5 |
H |
13.7g |
F |
7.92g |
1e |
54 |
Ash grey |
98 |
0.53
|
|
6 |
H |
13.7g |
Br |
12.3g |
1f |
40 |
Ash grey |
194-198 |
0.42 |
|
7 |
Br |
21.7g |
H |
7.03g |
1g |
62.6 |
Yellow |
184-188 |
0.54 |
|
8 |
Br |
21.7g |
Cl |
8.70g |
1h |
68.4 |
Yellow |
127 |
0.24
|
|
9 |
Br |
21.7g |
NO2 |
9.20g |
1i |
62.9 |
Yellow |
118 |
0.37
|
|
10 |
Br |
21.7g |
OCH3 |
8.55g |
1j |
43 |
Yellow |
178 |
0.46 |
|
11 |
Br |
21.7g |
F |
7.92g |
1k |
45 |
Ash grey |
122 |
0.43 |
|
12 |
Br |
21.7g |
Br |
12.3g |
1l |
38 |
Ash grey |
118 |
0.47 |
Step-II, Synthesis
A 0.05 Molar concentration of product (I) and hydrazine hydrate in ethanol was refluxed for three hours after that cooled. Ethanol was used to recrystallize the isolated solid (S). The melting points were. 1480C, 1450C, 1790C, 1580C, 1790C, 1440C, 1480C, 1400C, 1480C, 1430C,1400Cand1760C and yield were Compounds with 76%, 75%, 50, 56%, 54%, 50%, 65%, 71%, 64%, 44.9%, 43%, 44%, and 36% IIa-l, accordingly. “The values are IR (KBr in cm-1)” 3068 (C-H, ArHStr), 3305 (N-H Str), 1664 (cyclic C=0 Str), 1598 (C=N Str), 1514 (C=C Str), 1450 (C-N Str), and 839 (C-H deflaction). 1HNMR (ppm) (CDCl3), 4.530 (s, 3H, -SCH3), 7.484-8.090 (m, 8H, Ar-H), 3.78 (Methoxy proton ),1.7 (Methylene Proton ).4.07(3H, CH3) 11.32(s1H,NH).
The physical characteristics of synthesized final compounds
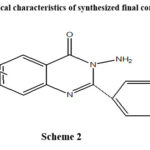 |
Scheme 2 |
Table 2
|
S.No. |
Code |
Name of the compound |
Quantity |
Yield (%) |
Color |
m.p. (0C) |
Rf |
|
1 |
2a |
3-aminoTwo-phenyl quinazolin-4(3H)-one |
2.38g |
26 |
Grayish |
147-150 |
0.59
|
|
2 |
2b |
three Amino Two-(4-chlorophenyl) quinazolin-4(3H)-one |
2.72g |
70 |
Grayish |
144-148 |
0.12 |
|
3 |
2c |
three Amino Two-(4-nitrophenyl) quinazolin-4(3H)-one |
2.83g |
28 |
Yellow |
178-182 |
0.2 |
|
4 |
2d |
three Amino Two-(4-methoxy phenyl) quinazolin-4(3H)-one |
2.68g |
56 |
Grayish |
156-164 |
0.37 |
|
5 |
2e |
three Amino Two-(4-flurophenyl) quinazolin-4(3H)-one |
2.55g |
52 |
Grayish |
177-180 |
0.44 |
|
6 |
2f |
three Amino Two-(4-bromophenyl) quinazolin-4(3H)-one |
3.17g |
38 |
Grayish |
182 |
0.36 |
|
7 |
2g |
6- bromo, 3-aminoTwo-phenyl quinazolin-4(3H)-one |
3.17g |
65 |
Yellow |
148 |
0.46 |
|
8 |
2h |
6- bromo, three Amino Two-(4-chlorophenyl) quinazolin-4(3H)-one |
3.82g |
71 |
Grayish |
120 |
0.32 |
|
9 |
2i |
6- bromo, three Amino Two-(4-nitrophenyl) quinazolin-4(3H)-one |
3.62g |
64 |
Yellow |
148 |
0.38 |
|
10 |
2j |
6- bromo, three Amino Two-(4-methoxy phenyl) quinazolin-4(3H)-one |
3.48g |
42 |
Grayish |
137-146 |
0.26 |
|
11 |
2k |
6- bromo, three Amino Two-(4-flurophenyl) quinazolin-4(3H)-one |
3.35g |
39 |
Grayish |
120 |
0.38 |
|
12 |
2l |
6- bromo, three Amino Two-(4-bromophenyl) quinazolin-4(3H)-one |
3.97g |
36 |
Grayish |
176 |
0.34 |
Anticonvulsant Activity Determination
Using the MES (maximal electroshock) method, we conducted assessments on the anti-convulsant activity of all synthesized compounds. Swiss albino mice, weighing between 20 and 35g and sourced from Bhubaneshwar, were employed in the study. The animal facility maintained a 12:12 h light/dark cycle, a room temperature of 24°C, humidity levels between 45% and 55%, and adhered to stringent hygiene standards for water supply. Prior to experimentation, the Institutional Animal Ethical Committee (IAEC) of SOA University Bhubaneswar granted approval in accordance with the ethical guidelines outlined by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), registration number 1171/c/08/(CPCSEA).Methodology involved the formation of three groups of male albino mice, each weighing 20–30g, with six animals in each group. The groups received different treatments: a control group (3% saline), a standard medicine group (phenytoin 25 mg/kg), and a group administered with the synthesized substance. For the experimental technique, mice were subjected to a 0.2-second electrical stimulus via small alligator clips attached to the cornea. A rectangular plastic cage with an open top was used for housing mice during the test session, allowing the recording of various parameters and full visibility of the animal’s motor reactions to seizures. During the 30-minute testing period, parameters such as clonus convulsions, tonic flexion, tonic extension, stupor, and the percentage of protection were recorded. Mean SEM values were calculated from the results of six animals. Statistical analysis involved ANOVA and Dunnett’s t-test to determine any significant differences between the groups, with a significance level set at P<0.05.19,20
Results are shown in (Table)
Table 3: Anticonvulsant characteristics of a synthesized compound
|
Treatment |
Dose |
Mean SD of the tonic hind limb extension time in seconds |
% Convulsion Inhibition |
Recovery |
|
Control |
2. ml/kg |
16.84 +/- 1.83 |
00.00% |
YES |
|
Standard |
25 mg/kg |
6.32 +/- 1.76 * |
63.5% |
YES |
|
A-1 |
50 mg/kg. |
7.54 +/- 1.73* |
55.5% |
YES |
|
A-2 |
50 mg/kg. |
14.26+/- 1.72 |
16.7% |
YES |
|
A-3 |
50 mg/kg. |
15.76 +/- 1.34 |
6.96% |
YES |
|
A-4 |
50 mg/kg. |
10.26+/- 0.99* |
38.7% |
YES |
|
A-5 |
50 mg/kg. |
14.34+/- 0.98 |
15.9% |
YES |
|
A-6 |
50 mg/kg. |
11.34+/- 1.50* |
33.7% |
YES |
|
B-1 |
50 mg/kg. |
14.7+/- 2.64 |
12.8% |
YES |
|
B-2 |
50 mg/kg. |
11.26+/- 0.99* |
33.6% |
YES |
|
B-3 |
50 mg/kg. |
16.26+/- 1.74 |
9.9% |
YES |
|
B-4 |
50 mg/kg. |
16.43+/- 1.52 |
2.9% |
YES |
|
B-5 |
50 mg/kg. |
11.2+/- 0.99* |
33.6 |
YES |
|
B-6 |
50 mg/kg. |
12.53 +/- 0.33* |
25.6% |
YES |
Result and Discussion
The synthesis of novel quinazolinone derivatives was achieved through a systematic multi-step process, involving the condensation of appropriate precursors. The structures of the synthesized compounds were confirmed through comprehensive spectroscopic characterization, including NMR and mass spectrometry. The successful synthesis of these compounds provided a solid foundation for further investigation into their pharmacological activities. The anticonvulsant potential of the synthesized quinazolinone derivatives was assessed using the Maximal Electroshock (MES) method, a standard preclinical model for screening antiepileptic drugs. Rodent models subjected to maximal electroshock-induced seizures were administered with various doses of the synthesized compounds, and their effects on seizure activity were meticulously observed.21-24
A glance at the table, which lists the anti-seizure activity and associated structures, reveals that almost all of the drugs are active. “The values are IR (KBr in cm-1)” 3068 (C-H, ArHStr), 3305 (N-H Str), 1664 (cyclic C=0 Str), 1598 (C=N Str), 1514 (C=C Str), 1450 (C-N Str), and 839 (C-H deflaction). 1HNMR (ppm) (CDCl3), 4.530 (s, 3H, -SCH3), 7.484-8.090 (m, 8H, Ar-H) 3.78 (Methoxy proton),1.7 (Methylene Proton ).4.07(3H, CH3) 11.32(s1H,NH), The analytical calculation of Compounds A-1(Carbon 66.46 %), (Hydrogen 5.99%),(Nitrogen 15.05%),A-3(Carbon 67.57%), (Hydrogen 7.88%),(Nitrogen 10.37%),B -4(Carbon 63.88 %),(Hydrogen 5.64%) , (Nitrogen 12.78 %), Mass Spectra of A-1,A-3 and B-4 m/z: 238, 282 and 397 respectively.25-27
The compounds B-4 and A-3, which were the least active, had the following chemical structures:
B-4 → R=Br
R’=OCH3
A-3 → R=H
R’=NO2
If we compare the structures of the produced compounds to their activities, we observe that the R’ group of the = C6H5- R’ should be substituted at position -4 of the ring. The activity is at its highest when the substitution is R= H, R’= H relative to other substituent’s.
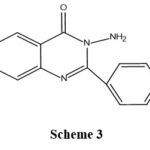 |
Scheme 3 |
The compound’s structure which gave the best activity is A-1
A-1→ R= H
R’= H
The unsubstituted compound showed the maximum activity then substituted compounds.
Conclusion
In conclusion, the synthesis and evaluation of novel quinazolinone derivatives for their anticonvulsant activity have yielded significant insights into their potential therapeutic applications. The diverse biological properties historically associated with quinazolinones have been further extended by the discovery of promising anticonvulsant effects in our study. The Maximal Electroshock (MES) method served as a robust preclinical model to assess the efficacy of the synthesized compounds in mitigating seizures. The observed anticonvulsant activity of select quinazolinone derivatives underscores their potential as candidates for further development in the treatment of epilepsy. The structure-activity relationships deduced from this research provide a foundation for future medicinal chemistry endeavors. Understanding the specific structural features that contribute to anticonvulsant effects is crucial for the rational design of more potent and selective compounds. Additionally, further studies exploring the mechanisms of action, pharmacokinetics, and safety profiles of these derivatives will be essential for their progression toward clinical applications.
Acknowledgment
All the authors are thankful to IES University, Bhopal for their valuable support.
Conflicts of Interest
There are no financial or other conflicts of interest that the authors of this work have disclosed.
Ethical Approvals
According the strict guidelines and ethical standards set out by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), registration number 1171/c/08/(CPCSEA), all procedures and experiments were conducted.
References
- Samira,I.; Patel,S.;.Hasmin, M.; Patel. S.; Int J Pharm Chem Sci. 2012,1,1863–1872
- Singh, V.K.; Singh, S.K.; Gangwar L. Int J Sci Res. 2013,2,425–428
- Ghorab, M.M.; Ismail, Z.; Radwan, A.A.; Acta Pharm. 2013,63,1–18.
CrossRef - Deep, A.; Narasimhan, B.; Ramasamy, K.; Mani, V.; Curr Top Med Chem. 2013,13,2034–2046
CrossRef - Al-Amiery, A.A.; Kadhum, A.Ah.; Shamel, M.; Satar, M.; Khalid, Y.; Mohamad, A.B.; Med Chem Res.2014,23,236–242
CrossRef - Laddha, S.S.; Wadod, S.G.; Meghal,.Sk.; Arkivoc. 2006,11,1–20
CrossRef - Krishnan, S.K.; Ganguly, S.; Veeramy, R.; Jan, B.;2011,15,673–681
- Dohle ,W.; Jourdan, F.L.; Menchon, G.; Prota, A.E.; Foster, P.A.; Mannion, P.;Journal Of Medicinal Chemistry. 2018,61,1031-1044
CrossRef - Jiang, S.; Zeng Q.; Gettayacamin, M.; Tungtaeng, A.; Wannaying, S.; Lim ,A.;Antimicrob Agents Chemoter. 2005,49,1169–1176
CrossRef - Hassanzadeh ,F.; Jafari. E.; Hakimelahi, G.H.;. Res Pharm Sci.2012,7,87–94.
- Giri, R.S.; Thaker, H.M.; Giordano, T.; Williams, J.; Rogers, D.;. Eur J Med Chem. 2009;44,184–2189.
- Samira, I.; Patel, S.; Hasmin, M.; Patel, S.; Int J Pharm Chem Sci.2012,1,1863–1872.
- Rajasekaran A, Rajamanickam V, Darlinquine S. Synthesis Of Some New Thioxoquinazolinone Derivatives And A Study On Their Anticonvulsant And Antimicrobial Activities. Eur Rev Med Pharmacol Sci.2013;17:95–104.
- Raffa, D.; Edler, M.C.; Daidone, G.; Maggio, B.; Merickech, M.; Plescia, S.; Eur J Med Chem.2004,39,299–304.
CrossRef - Grigg, R.; Mitchell, T.Rb.;J. Chem Soc Chem Commun. 1981,12, 1039
CrossRef - Maja, Molnar, Tatjana. Gazivoda, Kraljević, Valentina, Pavić, Vesna ,Rastija, Mario,Chemistry & Biodiversitychemistry & Biodiversitychemistry & Biodiversity, 2023, 20,8.
CrossRef - Davood, Gheidari, Morteza. Mehrdad, Saloomeh, Maleki, Chemistry And Pharmacy, 2022,10, 27
CrossRef - Rghavendra, N.M.; Asian Journal Of Chemistry, 2007,17, 57-65
- Afrah, Sharaf, Sherif, Shaban, Ragab, Ahmed, A.;Journal Of The Iranian Chemical Society, 2021,19,1, 291-302
CrossRef - Rajput, R.; And Mishra, A. P..;International Journal Of Pharmacy And Pharmaceutical Sciences, 2012, 4, 2, 66–70
- Raghavendra, N.M.; Thampi, P.; Gurubasavarajaswamy, P.M.; Sriram, D.: Chem. Pharm. Bull. 2007, 55, 1615-1619.
CrossRef - Ager, R.; Harrison, D.R.; Kennwell, P.D.; Taylor, J.B. J.Med. Chem. 1977, 20, 379-386.
CrossRef - Wolfe, J.F.; Rathman ,T.L.; Sleevi, M.C.; Campbell, J.A.; Grenn Wood ,T.D.. J. Med. Chem.1990, 33, 161-166.
CrossRef - Toman, J.E.P. Animal Techniques For Evaluating Anticonvulsants..; Year Book,Medical Publishers Inc.: Chicago, Il, Usa, 1964.
- Erin, Gordey; Paras, N. Yadav.; Marcus, P. Merrin.; Bioorganic & Medicinal Chemistry Letters, 2011,21, 4512-4515.
CrossRef - Paras ,N. Yadav.; Ramsay, E. Beveridge .; Jonathan ,Blay.; Med. Chem. Commun., 2011,2, 274-277
CrossRef - Paras ,N. Yadav.; Ramsay, E. Beveridge .; Jonathan ,Blay.; Trangition Medicinal Chemistry 1999,24,642-647
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









