Solvent Free Green Synthesis f 4-Substituted Thiocarbamido- Naphthols
Kiran P. Jumde1 , Sanghapal S. Padhen2*
, Sanghapal S. Padhen2* , Saifuddin. E. Quazi3
, Saifuddin. E. Quazi3 , Ruqquaiya Shaikh3
, Ruqquaiya Shaikh3 and Saleem. R. Khan3
and Saleem. R. Khan3
1Department of Chemistry, Nilkanthrao Shinde Science and Arts College, Bhadrawati (M.S.), India.
2Department of Chemistry, Rajarshee Shahu Science College, Chandur Railway, Dist Amravati. (M.S.), India.
3Department of Chemistry, Government Vidarbha Institute of Science and Humanites, Amravati (M.S.), India.
Corresponding Author E-mail: sangha0604@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400236
Article Received on : 01 Jan 2024
Article Accepted on :
Article Published : 11 Mar 2024
Reviewed by: Dr. Shivam Mishra
Second Review by: Dr. Naresh Batham
Final Approval by: Dr. Ravindra M Kumbhare
Recently in this laboratory we developed new solvent free green synthetic methods for the synthesis of 4-phenylthiocarbamido-1-naphthol, 4-(p-chloro)phenylthiocarbamido-1-naphthol, 4-(o-methyl)phenylthiocarbamido-1-naphthol, 4-(p-methyl)phenylthiocarbamido-1-naphthol, and 4-(m-methyl)phenylthiocarbamido-1-naphthol by interacting 4-amino-1-naphthol with p-chlorophenylisothiocynate, p-tolylisothiocynate, o-tolylisothiocynate and m-tolylisothiocynate in presence of sweet lemon juice, lemon and orange juice respectively which are hither to unknown. Yet such attempts were not carried out by any researcher for 4-amino-1-naphthol. Previous researchers carried out such type of synthesis for different molecules in organic solvents as medium, which are hazardous and make pollution of environment. Newly synthesized substituted thiocarbamidonapthols was characterized by elemental analysis, chemical characteristics and spectral studies.
KEYWORDS:Green synthesis; 4-substituted thiocarbamido naphthols
Download this article as:| Copy the following to cite this article: Jumde K. P, Padhen S. S, Quazi S. E, Shaikh R, Khan S. R. Solvent Free Green Synthesis f 4-Substituted Thiocarbamido-Naphthols. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Jumde K. P, Padhen S. S, Quazi S. E, Shaikh R, Khan S. R. Solvent Free Green Synthesis f 4-Substituted Thiocarbamido-Naphthols. Orient J Chem 2024;40(2). Available from: https://bit.ly/4a1u6ug |
Introduction
The literature survey reveals that several modern theories and concept concerning to the physical as well as chemical study of benzenoid, nonbenzenoid, heterocycles and heterocycles these compounds have their own individuality1-5. These compounds showed remarkable medicinal, biochemical, industrial, agricultural and pharmaceutical sciences. Thiocarbamido nucleus showed antimicrobial property as well as it acts as a versatile reagent in organic synthesis. Now-a-days thiocarbamido nucleus containg molecules showed great significances and scientific interest due their biological reactivity and biological features6-13. Various derivatives of this group have useful applications, some of these showed anti-tuberculosis, anti-tumor, anti-cancer, and anti-pyretic activities14-22. Considering all these things present research work is carried out solvent free green synthesis to synthesized new series of substitutedthiocarbamidonaphthols by the interaction of 4-amino-1-naphthol with phenylisothiocynate, p-chlorophenylisothiocynate, p-tolylisothiocynate, o-tolylisothiocynate and m-tolylisothiocynate in presence of lemon juice, sweet lemon juice and orange juice respectively which are hither to unknown. Scheme of synthesis is given in Scheme-I.
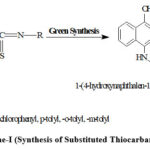 |
Figure 1: Scheme-I (Synthesis of Substituted Thiocarbamido Naphthols). |
Experimental Part
Glassware
All glassware used throughout the research work is of Borosil make.
Chemicals
India make all chemicals were used of AR grade of Merck and Aldrich. Perkin-Elmer spectrum RXI FTIR was used for IR Spectra and Bruker DRX-300 spectrometer operating at 300 MHz was used for 1H NMR spectra and solvent used CDCl3. Characterization was done at SAIF, Chandigarh, Punjab.
Method, Result and Discussion
4-(p-Methyl)phenylthiocarbamido-1-naphthol
Solvent freeinteraction of 4-amino -1-naphthol (0.1 M) and p-tolylisothiocynate (0.1 M) was carried out in sun light in presence of lemon juice (15 ml) which gave ivory colour needle shaped crystalline product. It was recrystallized by ethanol. Yield is 92.00% and melting point is 187-188ºC. Same interactions were carried out in sweet lemon juice as well as orange juice to check the percentage of yields and purity.
Properties
Compound is ivory colour crystalline solid. Compound having melting point 187-188ºC. Test for nitrogen and sulphur was positive. Presence of C=S group conform on the basis of positive alkaline plumbite test. Product was soluble in acetone, ethanol, benzene, dioxane, DMSO, DMSOd6 and water insoluble.
IR spectrum (cm-1)
KBr pallets was used for IR spectrum of compound. The essential absorptions are correlated as: N-H stretching at 3295.52cm-1, Phenolic-OH stretching at 1282.82 cm-1, Aromatic C-H stretching at 3059.54 cm-1, Aliphatic C-H stretching at 2969.54 cm-1, N-C-N stretching at 1605.81 cm-1, C-N stretching at 1520.94 cm-1, C-C stretching at 1390.74 cm-1, C-O stretching at 1282.72 cm-1, N-C=S stretching at 1520.94 cm-1, C=S stretching at 912.37 cm-1.
PMR spectrum
DMSO was used for the PMR spectrum of a compound. Due to presence of naphthol ring this spectrum distinctly displayed the signals six protons at δ 8.2072- 7.0867 ppm, Ar-H protons at δ 6.9071- 6.6549 ppm, phenolic –OH proton at δ 5.4632-5.2534 ppm, NH protons at δ 3.3706 ppm, and CH3 protons at 1.4823-1.2406.
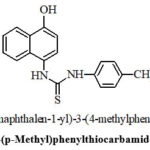 |
Figure 2: 4-(p-Methyl)phenylthiocarbamido-1-naphthol |
Synthesis of 4-phenylthiocarbamido-1-naphthol
Solvent free interaction of 4-amino-1-naphthol (0.1 M) and phenylisothiocynate (0.1 M) was carried out in lemon juice (15 ml) in presence of sun light which gave yellow colour needle shaped crystalline product. It was recrystallized by ethanol. Yield is 91.41% and melting point is 203-204ºC. Same reactions were carried out in sweet lemon juice as well as orange juice to check the percentage of yields and purity.
Properties
Compound isyellow needle shaped crystalline solid. It having melting point 203-204ºC. Test for nitrogen and sulphur was positive. Presence of C=S group conform on the basis of positive alkaline plumbite test. Product was soluble in acetone, ethanol, benzene, dioxane, DMSO, DMSOd6 and water insoluble.
IR spectrum (cm-1)
KBr pallets was used for IR spectrum of compound. The essential absorptions are correlated as: N-H stretching at 3171.11 cm-1, phenolic–OH stretching at 1598.09 cm-1, aromatic C-H stretching at 2971.47 cm-1, aliphatic C-H stretching at 2745.79 cm-1, N-C-N stretching at 1598.09 cm-1, C-N stretching at 1368.88 cm-1, C-C stretching at 1598.09 cm-1, C-O stretching at 1275.97 cm-1, N-C=S stretching at 1275.97 cm-1, C=S stretching at 1159.27 cm-1.
PMR spectrum
DMSO was used for the PMR spectrum of a compound. This spectrum distinctly displayed the signals due to Ar-H protons at δ 6.9071- 6.6549 ppm, naphthol ring six protons at δ 8.2072- 7.0867 ppm, phenolic –OH proton at δ 5.4632-5.2534 ppm, NH protons at δ 3.3706 ppm, and CH3 protons at 1.2406.
The compound was assigned the structure as 4-phenylthiocarbamido-1-naphthol, from the above chemical characteristics, elemental and spectral analysis.
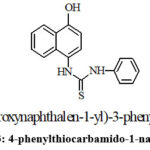 |
Figure 3: 4-phenylthiocarbamido-1-napthol |
Similarly, solvent free interactions of 4-amino-1-naphthol with p-chlorophenyl- isothiocynate, p-tolylisothiocynate, o-tolylisothiocynate and m-tolylisothiocynate in presence of sweet lemon juice, sweet lemon juice and orange juice were carried out in sun light respectively and results are listed in following.
Table 1
|
Sr. No. |
4-Substituted thiocarbamido-1-naphthol |
Melting Point (ºC) |
Yield in lemon Juice (%) |
Yield in Sweet lemon Juice (%) |
Yield in orange Juice (%) |
|
1 |
4-(p-Methyl)phenyl– |
187-188 |
92.00 |
——— |
——— |
|
2 |
4-(p-Methyl)phenyl– |
187-188 |
——— |
91.50 |
——— |
|
3 |
4-(p-Methyl)phenyl– |
187-188 |
——— |
——— |
91.50 |
|
4 |
4-Phenyl——– |
203-204 |
91.41 |
———- |
——— |
|
5 |
4-Phenyl——– |
203-204 |
———- |
90.20 |
———- |
|
6 |
4-Phenyl——– |
203-204 |
———- |
———- |
89.90 |
|
7 |
4-(p-Chloro)phenyl– |
217-218 |
90.00 |
———- |
——— |
|
8 |
4-(p-Chloro)phenyl– |
217-218 |
———- |
87.53 |
———- |
|
9 |
4-(p-Chloro)phenyl– |
217-218 |
———- |
———- |
87.00 |
|
10 |
4-(o-Methyl)phenyl– |
167-168 |
92.68 |
———- |
——— |
|
11 |
4-(o-Methyl)phenyl– |
167-168 |
———- |
90.00 |
———- |
|
12 |
4-(o-Methyl)phenyl– |
167-168 |
———- |
———- |
90.00 |
|
13 |
4-(m-Methyl)phenyl- |
175-176 |
91.50 |
———- |
——— |
|
14 |
4-(m-Methyl)phenyl- |
175-176 |
———- |
89.88 |
———- |
|
15 |
4-(m-Methyl)phenyl- |
175-176 |
———- |
———- |
89.80 |
Conclusion
It is observed from the literature survey, in our research group different type of thiocarbamide were synthesized by using acetone23-24 and acetone-ethanol (1:1) medium. It is also observed from the literature survey 5-thiocarbamide naphthol were synthesized in acetone medium25, but green synthetic methods are not investigated yet. The beauty of this research work and article said that in our synthetic method green synthesis parameter were maintain. It means that we didn’t use any type hazardous organic solvent as medium as well as we don’t use artificial heating condition means in our synthesis acetone-ethanol medium are not used and refluxion of the reaction mixture steps is also removed. Means all these reaction as well as synthesis as eco-friendly and protect the environment. It is again observed from the literature survey that the particular product yield are increase also purity of product was maintained. These molecules often have interesting biological activities.
Acknowledgement
The authors extend their heartful thanks to Dr. D.T. Tayade, Professor, Department of Chemistry, Government Vidarbha Institute of Science and Humanites, Amravati (M.S.) 444 604, India for moral support, guidance and valuable suggestions. Also thanks to SAIF, Chandigarh, Punjab for characterization.
Conflicts of Interest
The publication of this research article authors declare that have no conflict of interest.
References
- Panpalia.R.C, Ph.D. Thesis.S.G.B. Amravati University Amravati, 2007.
- Shedlovsky.T, J.Am.Chem.Soc., 1930, 52, 1793-1805.
CrossRef - Shedlovsky.T., J.Am.Chem.Soc., 1930, 52, 1806-1811.
CrossRef - Kohlrausch.F, Das.Malty and M.E, “Elektrische Leitvermogenwassriger, Losungevon, Alkali-Chloriden and Nitraten. Wiss. Abh. Phys. Tech. Reichsanst, 1900, 3, 155-228.
- Debye.P and Huckel.E, Theory of Electrolyte.11, The limiting law of electrical conductivity. Phys. Z., 1923, 24,305-325.
- Murshid.G and Garg.S.Initial solubility and density evaluation of Non Aqueous System of amino acid salts for CO2 capture: potassium prolinate blended with ethanol and ethylene glycol. IOP Conference Series: Earth and Environmental Science, 2018, 154(1), 12020.
CrossRef - Bian.Yand Shen.S. CO2 absorption into a phase change absorbent: Water-lean potassium prolinate/ethanol solution. Chinese Journal of Chemical Engineering, 2018, 26(11), 2318-2326.
CrossRef - Shen.S, Yang.Y.N, Bian.Y and Zhao.Y, Kinetics of CO2 absorption into aqueous basic amino acid salt: potassium salt of lysine solution. Environmental science and technology, 2016, 50(4), 2054-2063.
CrossRef - Mazinani.S, Ramazani.R, Samsami.A, Jahanmiri.A, Van der Bruggen.B and Darvishmanesh, S.Equilibrium solubility, density, viscosity and corrosion rate of carbon dioxide in potassium lysinate solution. Fluid Phase Equilibria, 2015, 396, 28-34.
CrossRef - Kumar.P.S, Hogendoorn.J.A, Feron.P.H.M and Versteeg.G.F Density, viscosity, solubility, and diffusivity of N2O in aqueous amino acid salt solutions. Journal of chemical & engineering data, 2001, 46(6), 1357-1361.
CrossRef - Wei, S. C. C., Puxty, G., and Feron, P. Amino acid salts for CO2 capture at flue gas temperatures. Energy Procedia, 2013, 37, 485-493.
CrossRef - Harris.F, Kurnia.K.A, Mutalib, M.I.A and Thanapalan.M, Solubilities of carbon dioxide and densities of aqueous sodium glycinate solutions before and after CO2 absorption. Journal of Chemical & Engineering Data, 2009, 54(1), 144-147.
CrossRef - Aronu.U.E, Hartono.A and Svendsen.H.F, Density, viscosity and N2O solubility of aqueous amino acid salt and amine amino acid salt solutions. The Journal of chemical thermodynamics, 2012, 45(1), 90-99.
CrossRef - Seyyed H.S. and Leila M., Solvent free synthesis of amidoalkyl derivatives under green and convenient conditions, Journal of Heterocyclic Chemistry. 2021, 59(4), 695-703.
CrossRef - Zhang, Z.; Gao, X.; Wan, Y.; Huang, Y.; Huang, G.; Zhang, G. Ammonium Acetate-Promoted One-Pot Tandem Aldol Condensation/Aza-Addition Reactions: Synthesis of 2,3,6,7-Tetrahydro-1H-pyrrolo [3,2-c]pyridin-4(5H)-ones. ACS Omega, 2017, 2, 6844–6851.
CrossRef - Ahmadzadeh, M.; Zarnegar, Z.; Safari, J. Sonochemical synthesis of methyl-4-(hetero)arylmethylene isoxazole-5(4H)-ones using SnII-montmorillonite. Green Chem. Lett. Rev. 2018, 11, 78–85.
CrossRef - Dommaraju, Y.; Borthakura, S.; Prajapati, D. L-Proline-Catalysed One-Pot aza-Diels–Alder Reaction in Water: Regioselective Synthesis of Spiro (isoxazolo[5,4-b]pyridine-5,50 -pyrimidine) Derivatives. Synlett, 2018, 29, 1195–1198.
CrossRef - Bhagat, S.B.; Telvekar, V.N. NBS mediated protocol for the synthesis of N-bridged fused heterocycles in water. Tetrahedron Lett., 2017, 58, 3662–3666.
CrossRef - Bhat, P.; Shridhar, G.; Ladage, S.; Ravishankar, L. An eco-friendly synthesis of 2-pyrazoline derivatives catalyzed by CeCl3 ·7H2O. J. Chem. Sci., 2017, 129, 1441–1448.
CrossRef - Bakherad, M.; Keivanloo, A.; Gholizadeh, M.; Doosti, R.; Javanmardi, M. Using magnetized water as a solvent for a green, catalyst-free, and efficient protocol for the synthesis of pyrano[2,3-c]pyrazoles and pyrano[40,30:5,6]pyrazolo[2,3-d]pyrimidines. Res. Chem. Intermed., 2017, 43, 1013–1029.
CrossRef - Campos, J.F.; Scherrmann, M.-C.; Berteina-Raboin, S. Eucalyptol: A new solvent for the synthesis of heterocycles containing oxygen, sulfur and nitrogen. Green Chem. 2019, 21, 1531–1539.
CrossRef - Hülsey, M.J.; Yang, H.; Yan, N. Sustainable Routes for the Synthesis of Renewable Heteroatom-Containing Chemicals. ACS Sustain. Chem. Eng., 2018, 65, 5694–5707.
CrossRef - Tayade D.T., Ph.D. Thesis.S.G.B. Amravati University Amravati, 1996.
- Bhagwatkar R.A., Ph.D. Thesis.S.G.B. Amravati University Amravati, 2012.
- Wadekar A.B., Ph.D. Thesis.S.G.B. Amravati University Amravati, 2017.

This work is licensed under a Creative Commons Attribution 4.0 International License.









