In-vitro Anti-urolithiasis Activity Evaluation of Acalypha rhomboidea Leaf Extract
Jahnavi Bandla1 , Ashok Gorja2
, Ashok Gorja2 , Mobeen Syed1, Swetha Kappala1*
, Mobeen Syed1, Swetha Kappala1*
1Vishnu Institute of Pharmaceutical Education and Research, Narsapur-502313, Medak, Telangana, India.
2Gokaraju Rangaraju College of Pharmacy, Bachupally-500090, Hyderabad, Telangana, India.
Corresponding Author E-mail: swethakappala5@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400218
Article Received on : 18 Mar 2024
Article Accepted on :
Article Published : 22 Apr 2024
Reviewed by: Dr. Pushpendra Sharma
Second Review by: Dr. Rajat Das
Final Approval by: Dr. Abdelwahab Omri
The present study explored the in-vitro evaluation of the anti-urolithiasis activity of Acalypha rhomboidea leaf extract. The powdered plant material was extracted using the maceration technique with water as solvent. After the extraction, the extract was subjected to preliminary qualitative analysis to identify the presence of phytochemicals. The phytoconstituents present in the extract are reducing sugars, phenolic compounds, flavonoids, alkaloids, tannins, saponins, terpenoids, glycosides, etc. Later aqueous extract was evaluated for anti-urolithiasis activity using the titrimetry method and percentage dissolution was calculated. In this method, neeri was used as a standard drug. The plant extract showed 82% percentage dissolution of calcium oxalate crystals indicating that the plant was more efficient in dissolving calcium oxalate when compared to standard drug.
KEYWORDS:Acalypha rhomboidea; Aqueous Extract; Calcium Oxalate Crystals; % Dissolution; Maceration; Urolithiasis
Download this article as:| Copy the following to cite this article: Bandla J, Gorja A, Syed M, Kappala S. In-vitro Anti-urolithiasis Activity Evaluation of Acalypha rhomboidea Leaf Extract. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Bandla J, Gorja A, Syed M, Kappala S. In-vitro Anti-urolithiasis Activity Evaluation of Acalypha rhomboidea Leaf Extract. Orient J Chem 2024;40(2). Available from: https://bit.ly/49L4f9x |
Introduction
Kidney stones about 20% of people suffer from nephrolithiasis or urolithiasis, also referred to as kidney or renal stones. The components of most urinary stones are uric acid, cysteine, phosphates, and oxalates. Calcium oxalate (CaOx) makes up about 80% of these calculi. Urolithiasis refers to the formation of stones, also known as calculi, in any part of the urinary system, including the renal pyelocalyceal system, ureter, bladder, and urethra, sometimes urine calculosis. After prostatic disorders and urinary tract infections, it is the third most prevalent urinary tract illness in humans. There are four types of lithiasis based on location: ureter lithiasis (stones in the tubules that carry urine from the kidneys to the bladder), bladder lithiasis, urethral lithiasis, and renal lithiasis (nephrolithiasis) (stones in the tubule ejecting pee from the bladder outwards)1.
Calcium-containing stones can be composed of either calcium phosphate (5%) pure calcium oxalate (45%), or a combination of the two, with magnesium phosphate coming in last. Historically, medicinal plants have been a significant source of pharmaceuticals. It has been claimed that a few proprietary composite herbal formulations and medicinal plants can effectively treat renal calculi and prevent their recurrence while having few adverse effects. One of the primary components of deposits in the urinary system is calcium oxalate. Calcium oxalate crystallization is particularly interesting due to its biological significance as well as its theoretical implications2. There is still some confusion regarding the precise process that triggers the development of calcium oxalate stones. Several external factors influence the nucleation, development, and aggregation of different calcium oxalate hydrates in addition to an excess of calcium and oxalate concentrations.
Numerous investigations have been conducted to ascertain the impact of several additions, including metallic ions and their complexes. The predominant medical strategy for managing urolithiasis involves the removal of stones through endoscopic procedures. Although techniques such as extracorporeal shock wave lithotripsy (ESWL) have revolutionized this domain, they do not diminish the likelihood of future stone formation. They include adverse effects that include bleeding, high blood pressure, tubular necrosis, renal fibrosis, and an increased risk of stone recurrence. They are also costly.
According to data from clinical trials conducted both in vivo and in vitro, phytotherapeutic drugs may offer an alternate course of treatment for urolithiasis3. Exploration of traditional knowledge regarding medicinal plants can lead to the discovery and development of new drugs and pharmacologically valuable products for human healthcare. Compared to synthetic pharmaceuticals, which are expensive and frequently have negative effects, green medicines are more trustworthy and safe. Even while technological advances have significantly improved the treatment of urinary stones, there are still certain disadvantages to these procedures, such as the fact that they are too expensive for the average person and that stone formation can recur in addition to a host of other adverse effects4.
Plants of the Euphorbiaceae spurge family include Acalypha rhomboidea (Fig. 1). Rhomboidea, the species name, refers to the “diamond-shaped” nature of the leaves. Although it can withstand clay or gravelly soil, this plant prefers fertile loam soil. For birds like the larger prairie chicken and mourning dove, the seeds provide an alluring food source. It is known that deer browse Acalypha sp. Acalypha rhomboidea has medicinal benefits such as antioxidant, and anthelminthic activities5-7. The main aim is to evaluate the Acalypha rhomboidea leaf extract for in-vitro anti-urolithiasis activity.
 |
Figure 1: Acalypha rhomboidea plant |
Materials and Methods
Identification and collection of plant material
The leaves of Acalypha rhomboidea were collected from Khagazmaddur (Village), Narsapur (Mandal), Medak (District) of Telangana in September 2023. D. Venkateshwara Rao, Deputy Director, Telangana Forest Academy, Dulapally, Hyderabad, Rangareddy District, verified the authenticity of the plant. After being cleaned with tap water, the leaves were dried in the shade.
Chemicals
The chemicals utilized in this study, including sodium oxalate, tris buffer, calcium chloride, potassium permanganate, and sulfuric acid, were of analytical reagent (AR) grade and obtained from Merck, Mumbai. Standard drug (Neeri tablet) were purchased from the local pharmacy.
Extraction of plant material
To create powder, the plant’s dried leaves were ground up in a pulverizer. This plant material was macerated for 48 hours with water. Following the extraction process, the extracts were allowed to cool to room temperature before being filtered and dried using a rotary evaporator.
Preliminary phytochemical screening
To undertake chemical tests and qualitative analyses of phytoconstituents such as alkaloids, glycosides, phenolic components, flavonoids, tannins, saponins, steroids, carbohydrates, and terpenoids, etc, the aqueous extract was treated with a variety of chemical reagents8-10.
Alkaloids
A few drops of Mayer’s reagent were added to 1 ml of extract. The formation of a creamy white precipitate will be considered positive for the alkaloid.
Carbohydrates
Add 1 ml of the Molisch reagent to the extracts taken in test tubes and slowly introduce concentrated sulfuric acid with a gentle addition along the inner walls of the test tubes. The detection of a violet ring forming at the juncture between the two liquids signifies the existence of carbohydrates within the extract.
Reducing sugars
0.5 ml of plant extract, 1 ml of water, and 5-8 drops of Fehling’s solution were added and heated. The presence of reducing sugar will be indicated by the appearance of brick-red precipitation.
Steroids
Add 10 ml of chloroform to the extract, followed by the introduction of 10 ml of sulfuric acid. The presence of steroids is indicated if the upper layer displays a red color and the sulfuric acid layer exhibits a yellow-green shade.
Terpenoids
Combine 2 ml of chloroform with 1 ml of extract, and then add a small quantity of sulfuric acid. The emergence of a reddish-brown color at the boundary suggests the presence of terpenoids.
Flavonoids
Mix 1 ml of extract with 2 ml of 2N sodium hydroxide. The presence of a yellow color indicates the existence of flavonoids.
Tannins
In test tube of extract, introduce 2 drops of 10% lead acetate. The presence of reddish-brown precipitate points to the existence of tannins.
Saponins
About 0.5 ml of the extract and 5 ml of distilled water were combined and agitated. Then the formation of foam confirms the presence of saponins.
Glycosides
Adding 1 ml of distilled water and sodium hydroxide to 0.5 ml of crude extract, the formation of a yellowish color indicates the presence of glycosides.
Cardiac glycosides
Qualitative analysis of extract for cardiac glycosides was performed with the Keller Killiani test, in which 1 ml of acetic acid and 2 drops of ferric chloride were added to 2 ml of extract. Then 2 ml of concentrated sulfuric acid was added and the color change must be observed. The formation of a reddish-brown color indicates the presence of cardiac glycosides.
Anthraquinones
To extract, an equal volume of 10% ammonia solution is to be added and mixed. The appearance of a bright pink color in the upper aqueous layer indicates the presence of anthraquinones.
Anthocyanin
An aliquot of 1 ml of 2N sodium hydroxide is to be added to 2 ml of plant extract and heated at 100°C for about 5 min to assess the presence of anthocyanin. The formation of a bluish-green color indicates the presence of anthocyanin.
Coumarins
A volume of 1 ml of 10% sodium hydroxide is to be added to 1 ml of the plant extract. Yellow color formation confirms the existence of coumarins in the plant extract.
Phenolic compounds
About 1 ml of the extract will combined with three drops of ferric chloride and 1 ml of potassium ferricyanide. The formation of greenish-blue confirms the presence of phenols.
Evaluation of in-vitro anti-urolithiasis activity
In the laboratory, experimental kidney stones composed of Calcium Oxalate (CaOx) were produced by mixing equimolar solutions of calcium chloride and sodium oxalate in 10 ml of 2N sulfuric acid. When both were given enough distilled water to react in a beaker, the CaOx precipitate was the end product. Ammonia solution was used to remove any remaining sulfuric acid from the precipitate, which was then cleaned with distilled water and dried at 60°C.
Between the egg’s interior components, such as albumin and yolk, and its outer, calcified shell, is a semipermeable membrane. The eggs’ shell was removed by completely decalcifying them overnight in 10% acetic acid as shown in Fig. 2 and 3. Moreover, the top of the decalcified eggs is punctured with a sharp tip and thoroughly cleaned with distilled water to extract all of the contents. The egg membrane was then completely cleaned with distilled water, soaked for a while in an ammonia solution, and then rinsed with purified water. After that, keep at a pH of 7 to 7.4 in the refrigerator3.
By precisely combining In the model depicted below, 10 mg of calcium oxalate and 100 mg of the extract were placed within the semipermeable membrane of an egg in Fig. 4, the dissolving percentage of calcium oxalate was determined. This was left to float in 100 milliliters of 0.1M Tris buffer in a conical flask. The initial group was used as a blank and contained just 1 mg of CaOx. The second group functioned as a positive control and was administered 10 mg of the usual medication, Neeri, coupled with 1 mg of CaOx. Aqueous extract is included in the third group along with 10 mg of calcium oxalate.
The conical flasks from all groups were placed into an incubator preheated to 37°C for two hours. Each group’s semipermeable membrane contents should be taken out and placed into a different test tube. Next, each test tube should contain 2 milliliters of 1N sulfuric acid, and 0.9494 N potassium permanganate should be added until a bright pink endpoint is reached. To ascertain the overall quantity of dissolved calcium oxalate through various solvent extractions, the initial amount utilized in the experiment is subtracted from the residual undissolved calcium oxalate remaining4.
The calculation for dissolved calcium oxalate involves subtracting the undissolved calcium oxalate from the total quantity used in the experiment initially. The percentage dissolution is determined by multiplying the dissolved calcium oxalate by 100.
Dissolved Calcium oxalate = (total quantity used in the experiment in the beginning – undissolved calcium oxalate)
Percentage dissolution = Dissolved Calcium oxalate × 100
Results and Discussion
After the collection and identification of the plant, the leaves were cleaned and dried. Later the dried leaves were ground to powder and extracted with aqueous solvent using maceration technique. Next, the acquired extract underwent an initial screening to identify different constituents using phytochemical methods.
Anthraquinones, anthocyanins, coumarins, phenolics, flavonoids, alkaloids, tannins, terpenoids, saponins, cardiac glycosides, and reducing sugars were found in the aqueous extract according to the preliminary phytochemical screening as shown in Table 1. Phytochemicals like terpenoids, tannins, alkaloids, and saponins may be what provides plant extract its anti-urolithiasis properties11.
Table 1: Phytochemical screening for constituents
|
Chemical constituents |
Aqueous extract |
|
Alkaloids Carbohydrates Glycosides Phenolics Steroids Proteins Terpenoids Flavonoids Coumarins Resins Tannins Saponins |
+ + + + – + + + + – + + |
(+) indicates present and (-) indicates absent
After performing the phytochemical analysis, the plant extract was subjected to assess the anti-urolithiasis activity of the Acalypha rhomboidea. The assessment was carried out by using the titrimetry method, in which artificial kidney stones from calcium oxalate were prepared and the percentage dissolution of stones was calculated.
 |
Figure 2: Eggshell decalcification was carried out overnight using a 10% acetic acid solution. |
 |
Figure 3: Decalcified Eggs. |
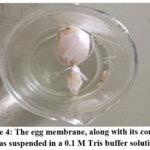 |
Figure 4: The egg membrane, along with its contents, was suspended in a 0.1 M Tris buffer solution |
The percentage dissolution was calculated for the blank, positive control (neeri), and the aqueous extract, and the results were summarized in Table 2. It was found that 82% calcium oxalate dissolution was observed in the aqueous extract whereas with positive control it was 81%. This shows that Acalypha rhomboidea leaf extract can be used successfully for the treatment of urolithiasis.
Table 2: % The dissolution of calcium oxalate (CaOx) was facilitated by extracts derived from Acalypha rhomboidea leaves.
|
S. No. |
Groups |
Acalypha rhomboidea |
|
1 2 3 |
Blank Positive control Aqueous extract |
0 81 82 |
Conclusion
We anticipated that this study would stimulate more research into novel medications for the management and prophylaxis of urolithiasis. The current studies offer important details regarding Acalypha rhomboidea leaves anti-urolithiatic activity. Some constituents like alkaloids, saponins, flavonoids, tannins, terpenoids, phenolic compounds, cardiac glycosides, reducing sugars etc were found after the phytochemical evaluation of the plant. The aqueous extract demonstrated the calcium oxalate stones disintegration by using titrimetric method analyzing the % dissolution of calcium oxalate crystals. Additional research in pharmacology and clinical settings is necessary to fully comprehend the workings and true effectiveness of the Acalypha rhomboidea plant in the treatment of urolithiasis.
Acknowledgment
The authors are also thankful to the management of VIPER for providing the facilities to perform the proposed work.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Kaleeswaran, B.; Ramadevi, S.; Murugesan, R.; Srigopalram, S.; Suman, T.; Balasubramanian, T. J. Tradit. Complement. Med. 2019, 9, 24-37.
CrossRef - Merin, B.; Uma, K.H.; Sherin, J.; Amoolya, S.; Sabin, S.; Shibina, K.A.; Jahanara, H. Biomed. Pharmacol. J. 2021, 14(2), 671-680.
CrossRef - Vithursha, S.; Paheerathan, V.; Senevirathne, AAI. J. Nat. Ayu. Med. 2023, 7(1), 000370.
- Srikaran, R.; Salika D.S. Int. J. Curr. Pharm. Res. 2019, 11(1), 18-20.
CrossRef - Layla, G.S.; Prabodh, S.; William, N.S. Am. J. Essent. Oil Nat. Prod. 2019, 7(3), 27-30.
- Purushotham, K.; Srikanth, I.; Mukhesh, N.; Swetha, K.; Ramanjaneyulu, K.; Himabindhu, J. J. Pharmacogn. Phytochem. 2019, 8(6), 915-917.
- Nyero, A.; Anywar, G.U.; Achaye, I.; Malinga, G.M. Front. Nutr. 2023, 10, 1070031.
CrossRef - Swetha, K.; Ashok, G.; Swathi, M.; Nameera, J.; Jahnavi, B. Orient. J. Chem. 2023, 39(5), 1321-1324.
CrossRef - Evans, W.C. Pharmacognosy. 1989, 33, 471.
- Harborne, A. Springer science & business media, 1998.
- Ravi Kumar, K.; Pratyusha, A. UPI J. Pharm. Med. Health Sci. 2020, 3(1), 5-8.

This work is licensed under a Creative Commons Attribution 4.0 International License.









