An Eco-friendly Corrosion Inhibitor: Neolamarckia Cadamba Leaves Extracts for SS 304 in 1 M HCl Solution
Department of Chemistry, Sankalchand Patel University, Visnagar, Gujarat, India.
Corresponding Author E-mail: dr.dhara29@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400229
Article Received on : 17 Feb 2024
Article Accepted on :
Article Published : 17 Apr 2024
Reviewed by: Dr. Rebaz Anwar Omer
Second Review by: Dr. Meena M
Final Approval by: Dr. Ravindra M Kumbhare
Neolamarckia Cadamba leaf extract as a green and environment-friendly inhibitor for the protection of stainless steel (SS) 304 from corrosion in 1 M HCl solution. The inhibition effect of NC inhibitor was measured by different electrochemical methods for example Weight loss measurement, SEM-scanning electron microscopy, PDP-potentiodynamic polarization, and EIS-electrochemical impedance spectroscopy used for the inhibition effects studies. Using Langmuir adsorption isotherm, we studied the adsorption of the extracts of leaves. All of the methods used indicate a productive result on inhibition efficiency with a concentration of natural inhibitor increasing.
KEYWORDS:Corrosion; Inhibitor; Stainless steel; Surface
Download this article as:| Copy the following to cite this article: Patel J, Patel D. D. An Eco-friendly Corrosion Inhibitor: Neolamarckia Cadamba Leaves Extracts for SS 304 in 1 M HCl Solution. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Patel J, Patel D. D. An Eco-friendly Corrosion Inhibitor: Neolamarckia Cadamba Leaves Extracts for SS 304 in 1 M HCl Solution. Orient J Chem 2024;40(2). Available from: https://bit.ly/3UmcnIY |
Introduction
Within manufacturing refining for example cooling systems, oil well acidizing, acid pickling, and chemical and electrochemical etching1. Some treatments of corrosion for stainless steel protection, and corrosion inhibitors because of their reasonable cost, simple utilization, and abundant productivity have attracted great attention recently2. Environmental concern and safety of corrosion inhibitors emerging in factories have been a worldwide issue3. Corrosion inhibitors are materials that form a layer of metal in a corrosive medium to slow metal corrosion4-5. In a past few days, the evolution of sustainable and environment-friendly corrosion inhibitors has been paid attention to by researchers6. Synthesized corrosion inhibitors are nitrogen, oxygen, and sulfur-containing organic compounds7-8. The polar groups of synthesized molecule structures can behave as working centers that play a major role in the absorption of the inhibitor on steel surfaces, throughout the synthesis process of organic inhibitor, the environment is polluted9-11. The inhibitive properties of plant extracts of Acalypha torta leaf12, Bamboo leaf13, Colocasia esculenta leaves14, Ficus tikoua leaves15, Geissospermum leaves16, Gingo leaves17, Glycyrrhiza glabra leaves18, Emblica officinalis leaves19, Dendrocalamus brandisii20, Olive leaf21, Salvia officinalis22 have been worked for the metal in various medium as green corrosion inhibitor. There is data on Neolamarckia Cadamba (NC) plant leaf extract as a green corrosion inhibitor, as an example, as a corrosion inhibitor neolamarckia cadamba is used for mild steel in 1 M HCl23. Here we use Neolamarckia Cadamba for stainless steel (SS) 304 in 1 M Hydrochloric acid solution. Neolamarckia cadamba is allied to the Rubiaceae family which has different remedial properties as an astringent, antidiuretic, and antihepatotoxic. Former studies of the Neolamarckia cadamba plant by phytochemical experiments have led to neolamarckines a and b, indole alkaloids identification and isolation24-26. Leaves extract of Neolamarckia cadamba was evaluated for stainless steel 304 in 1 M Hydrochloric acid medium by applying the following methods: SEM-scanning electron microscopy, PDP-potentiodynamic polarisation, and EIS-electrochemical impedance spectroscopy, adsorption behavior between corrosion inhibitor and metal surface and studied by Langmuir adsorption isotherm.
Experimental Methods
Material Details
SS 304 electrode was prepared from: the chemical composition of Fe, rest; Ni, 8.12; Cr, 18.06; Mn, 1.74; Si, 0.35; C, 0.065; S, 0.012; Mo, 0.149; Cu, 0.233; and P, 0.028 (wt%). Preparation of 1 M hydrochloric acid solution by hydrochloric acid 37% with purified water at ambient temperature.
Preparation of NC
The leaves of Neolamarckia cadamba were collected and cleaned with water, the NC leaf was seared in an oven at 373 K for the moisture content removal, then the heated leaf was grinded to a fine powder, then dried leaves were separately extracted with hexane, then soaked in 25% NH4OH for 2 hours, then they were moistened with CH2Cl2 twice over 3 days, dichloromethane crudes from leaves, from the leaves the crude alkaloid extracts were treated with acid-base extraction by using 5% hydrochloric acid and 25% ammonium hydroxide27.
Weight loss measurement
Stainless steel 304 weight loss calculations were executed in 1 M hydrochloric acid solutions and with and without inhibitors on stainless steel at different temperatures. Stainless steel samples were taken from the acidic medium after the immersion, cleaned with purified water, using acetone for cleaning, seared, and weighed. Difference in the sample weight was noted by weighing scale before and after immersion, -to confirm high precision in weighing, and to collect information of each inhibitor concentration, four sets of the same samples were used at different temperatures28.
Table 1: Values of weight loss
|
Temp. (K) |
Conc. of inhibitor (mg/l) |
Weight loss (g) |
|
298 |
0 |
0.038 |
|
25 |
0.019 |
|
|
50 |
0.014 |
|
|
75 |
0.011 |
|
|
100 |
0.008 |
|
|
303 |
0 |
0.041 |
|
25 |
0.021 |
|
|
50 |
0.016 |
|
|
75 |
0.012 |
|
|
100 |
0.010 |
|
|
308 |
0 |
0.045 |
|
25 |
0.023 |
|
|
50 |
0.019 |
|
|
75 |
0.014 |
|
|
100 |
0.011 |
Result and Discussion
SEM for Surface Analysis
SEM-Scanning electron microscopy shows an extremely magnified image of the plane of the metal, in SEM, the electrons are focused and scanned over the surface of a sample, so SEM analysis is regularly carried out with the absorption of the specimen in a corrosive medium with and without of inhibitor to examine the plane structure of steel29-33. Fig.1 indicates the surface structure of stainless steel plunged in 1 M HCl at room temperature with and without the inhibitor at various concentrations. To indicate other perceptions into the sample created by the chemisorption of a molecule of inhibitor on a stainless steel plane. As Fig.1 shows before immersion stainless steel had a smooth surface (Fig. 1A). Although, the sample corroded, damaged, and became a rougher and deeper crack in an acidic medium in the absence of an inhibitor caused by the steel cessation in corrosive medium and uneven plane constructed (Fig. 1B). In difference, when an inhibitor concentration increases, the surface of steel is improved in appearance observed in images of SEM (Fig. 1(C, D, E, F)). We observe the protective layer formation of inhibitors around the SS, which protects stainless steel against the acidic medium.
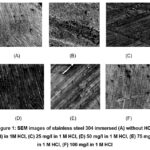 |
Figure 1: SEM images of stainless steel 304 immersed (A) without HCl, (B) in 1M HCl, (C) 25 mg/l in 1 M HCl, |
Potentiodynamic Polarisation measurements
Typical PDP for SS in 1 M hydrochloric acid carrying various NC inhibitor concentrations have been seen in Fig.2. The worth of electrochemical variables, for example, a current density of corrosion (Icorr), potential of corrosion (Ecorr), percentage of inhibition efficiency (η%) and cathodic and anodic tafel plots (βc and βa)34-36, which concluded from curves of polarization at different temperature like 298 K, 308 K and 318 K are listed in Table 1. Fig.2. exemplify the polarization plots of cathodic and anodic for stainless steel 304 in 1 M hydrochloric acid with and without concentration of NC inhibitor. As shown, the NC inhibitor addition shifted both cathodic and anodic lines, so the NC inhibitor was observed as a mixed type at each temperature. Table 1 indicates that when we increase inhibitor concentration, the current density of corrosion (Icorr)decreases. In addition to the NC inhibitor, the changes of cathodic (βc) and anodic (βa) tafel graphs observed, they recommended that the inhibitors in the corrosive media affected the cathodic and anodic kinetic processes.
Table 2: Potentiodynamic polarisation plots of electrochemical variables for stainless steel 304 in 1 M hydrochloric acid medium with and without concentrations of NC inhibitor at various temperatures.
|
T (K) |
C (mg/l) |
Ecorr (mV/ SCE) |
Icorr (μA cm-2 ) |
βa |
βc |
η% |
|
298 |
Blank |
-462 |
348 |
-100 |
81 |
– |
|
25 |
-460 |
134 |
-92 |
72 |
62 |
|
|
50 |
-467 |
102 |
-94 |
83 |
71 |
|
|
75 |
-464 |
75 |
-91 |
76 |
78 |
|
|
100 |
-468 |
48 |
-87 |
79 |
86 |
|
|
308 |
Blank |
-451 |
722 |
-70 |
82 |
– |
|
25 |
-456 |
141 |
-81 |
75 |
67 |
|
|
50 |
-453 |
108 |
-83 |
67 |
76 |
|
|
75 |
-452 |
88 |
-76 |
71 |
83 |
|
|
100 |
-455 |
75 |
-79 |
63 |
89 |
|
|
318 |
Blank |
-430 |
1240 |
-88 |
86 |
– |
|
25 |
-458 |
362 |
-86 |
75 |
71 |
|
|
50 |
-466 |
183 |
-95 |
77 |
82 |
|
|
75 |
-473 |
175 |
-114 |
79 |
87 |
|
|
100 |
-469 |
160 |
-105 |
82 |
91 |
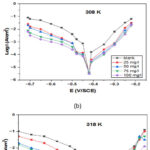 |
Figure 2: Polarisation plots of stainless steel 304 |
EIS measurements
EIS is a genuine and implied method for relaying corrosion inhibitors, EIS provides characteristics regarding surface properties and electrode kinetics process, and this method is significantly working for looking into the corrosion inhibition procedure37-38. In the study of electrochemicals we have to understand further techniques that are impedance spectroscopy, the study by EIS is extra precise for the inhibition efficiency of metal compared to the other techniques because it gives many variables of electrochemical exactly39-40. Fig.3 shows the Nyquist diagram of SS in 1 M hydrochloric acid containing various NC inhibitor concentrations at 298 K, 308 K, and 318 K separately. In the graph hemicycle indicates the radius of the hemicycle increases. When the concentration of NC inhibitor increases, the radius of the hemicycle increases. The radius of the hemicycle decreases with the temperature increase. Electrochemical variables for impedance such as resistance of charge transfer (Rct), solution resistance (Rs), capacitance of double layer (Cdl), the magnitude of CPE (Yo), deviation parameter (n), and inhibition efficiency (η%).
Table 3: Electrochemical variables of impedance for stainless steel 304 in 1 M hydrochloric acid solution of NC inhibitor with and without different concentrations at several temperatures.
|
T (K) |
C (mg/l) |
Rs (Ω cm2) |
Rct (Ω cm2) |
n |
Yo |
Cdl (μF cm−2 ) |
η% |
|
298 |
Blank |
1.3 |
68.4 |
0.92 |
415.6 |
342.6 |
– |
|
25 |
2.1 |
192.8 |
0.89 |
246.7 |
156.3 |
63.4 |
|
|
50 |
8.9 |
256.5 |
0.84 |
186.4 |
115.5 |
76.2 |
|
|
75 |
1.1 |
282.6 |
0.81 |
152.9 |
102.7 |
82.7 |
|
|
100 |
2.5 |
478.2 |
0.85 |
138.2 |
96.0 |
86.9 |
|
|
308 |
Blank |
4.1 |
29.8 |
0.83 |
824.5 |
424.3 |
– |
|
25 |
2.6 |
157.4 |
0.79 |
312.7 |
147.1 |
68.7 |
|
|
50 |
0.8 |
194.2 |
0.80 |
198.6 |
98.6 |
77.3 |
|
|
75 |
3.4 |
210.7 |
0.77 |
202.3 |
91.5 |
84.6 |
|
|
100 |
9.2 |
238.8 |
0.84 |
216.2 |
87.2 |
87.1 |
|
|
318 |
Blank |
0.9 |
23.6 |
0.94 |
776.5 |
505.6 |
– |
|
25 |
2.1 |
81.3 |
0.83 |
315.3 |
132.8 |
72.4 |
|
|
50 |
0.8 |
122.7 |
0.85 |
156.9 |
86.4 |
78.7 |
|
|
75 |
4.9 |
145.8 |
0.81 |
149.7 |
80.5 |
85.9 |
|
|
100 |
3.0 |
168.6 |
0.78 |
143.4 |
73.9 |
88.5 |
 |
Figure 3: Nyquist diagram for stainless steel 304 |
Langmuir Adsorption
The creation of an interchange process connecting corrosion inhibitor particles and the plane of metal is studied at the different isothermal adsorption techniques, molecular adsorption is described by the essential inhibition elements in the green inhibitors are commonly heterocyclic molecules, many adsorption isotherms were balanced which includes Temkin, Frumkin, Langmuir adsorption had an explain adsorption behavior by experimental data41-44. In this Langmuir adsorption isotherms study were used to report the adsorption types of NC inhibitors on the SS plane. Fig.4. shows a linear line of c/θ vs c at 25oC, 35oC, 45oC respectively. From the equation of Langmuir adsorption isotherm, when the value of Kads is higher, an NC inhibitor tightly adsorbs on the metal surface, which indicates a good inhibitive ability.
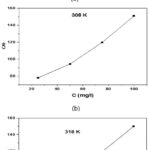 |
Figure 4: Langmuir adsorption for stainless steel 304 in 1 M HCl at various temperatures: (a) 298 K, (b) 308 K, and (c) 318 K |
Conclusion
In this study, Neolamarckia Cadamba leaf on stainless steel 304 in 1 M hydrochloric acid medium was resoluted by SEM, PDP, and EIS analysis. The result manifested that when inhibitor concentration increased, the corrosion rate decreased. The NC inhibitor adsorption on the stainless steel 304 surface obeyed the adsorption of langmuir and was associated with chemical adsorption and physical adsorption. SEM observation confirmed that the inhibition of NC derived that protective layer was carved on a stainless steel surface. Tafel polarization curves recommend that NC leaves extracts of confined stainless steel corrosion inhibitor as a mixed type. Thus this study illuminates the Neolamarckia Cadamba leaf extracts applied as a productive inhibitive solution of stainless steel 304 in 1 M HCl medium. So Neolamrckia Cadamba inhibitor is an earth-friendly, green, and productive corrosion inhibitor.
Acknowledgment
I am thankful to the Department of Chemistry, Sankalchand Patel University, Visnagar for providing all facilities for experimental work.
Conflict of Interest
The authors declare no conflict of interest.
References
- G. Golestani, M. Shahidi, and D. Ghazanfari, Appl. Surf. Sci., 2014, 308, 347–362.
CrossRef - A. Ehsani, M. G. Mahjani, M. Hosseini, R. Safari, R. Moshrefi, and H. Mohammad Shiri, J. Colloid Interface Sci., 2017, 490, 444–451.
CrossRef - P. B. Raja and M. G. Sethuraman, Mater. Lett., 2008, 62, 113–116.
CrossRef - Lana Omar Ahmed, Omer Kaygili, Niyazi Bulut, Rebaz Anwer Omer, Journal of Physical Chemistry and Functional Materials, 2023, 6(1), 34-42.
CrossRef - Lana Ahmed, Rebaz Omer, Journal of Physical Chemistry and Functional Materials, 2021, 4(2), 1-4.
CrossRef - E. Kowsari, Corros. Sci., 2016, 112, 73–85.
CrossRef - A. Yildirim and M. Çetin, Corros. Sci., 2008, 50,155–165.
CrossRef - N. M. Hashim, A. A. Rahim, H. Osman, and P. B. Raja, Chem. Eng. Commun., 2012, 199, 751–766.
CrossRef - R. Tambun, E. Christamore, Y. F. Pakpahan, and B. Haryanto, IOP Conf. Ser. Mater. Sci. Eng., 2018, 420.
CrossRef - C. Bala Manikandan, S. Balamurugan, P. Balamurugan, and S. L. Beneston, Int. J. Innov. Technol. Explor. Eng., 2019, 8, 1372–1375.
- A. Ismail and M. A. M. Tajuddin, Adv. Mater. Res., 2015, 1087, 282–286.
CrossRef - Pavithra M. Krishnegowda, Venkatarangaiah T. Venkatesha, Punith Kumar M. Krishnegowda, Shylesha B. Shivayogiraju, S. B. Shivayogiraju, Ind.Eng.Chem.Res., 2013, 52, 722-728.
CrossRef - X. Li, S. Deng, H. Fu, and X. Xie, Corros. Sci, 2013.
- N. Eddy and P. P. Mamza, Port. Electrochim. Acta, 2009, 27, 443-456.
CrossRef - Q. Wang, Bioelectrochemistry, 2019, 128, 49-55.
CrossRef - M. Faustin, A. Maciuk, P. Salvin, C. Roos, and M. Lebrini, Corros. Sci., 2014.
CrossRef - S. Deng and X. Li, 2012, 55, 407-415.
CrossRef - E. Alibakhshi, M. Ramezanzadeh, G. Bahlakeh, and B. Ramezanzadeh, J. Mol. Liq., 2018, 255, 185–198.
CrossRef - R. Saratha and V. G. Vasudha, E-Journal Chem., 2010, 7, 677–684.
CrossRef - X. Li and S. Deng, Corros. Sci., 2012, 65, 299–308.
CrossRef - M. Ben Harb, S. Abubshait, N. Etteyeb, M. Kamoun, and A. Dhouib, Arab. J. Chem., 2020, 13, 4846–4856.
CrossRef - N. Soltani, N. Tavakkoli, M. Khayatkashani, and M. Reza, Corros. Sci., 2012, 62, 122–135.
CrossRef - P. B. Raja, A. K. Qureshi, A. Abdul Rahim, H. Osman, and K. Awang, Corros. Sci., 2013, 69, 292–301.
CrossRef - D. Of, N. Cadamba, F. O. R. Sustainable, S. Of, M. For, and C. Plantation, 2015, 24, 159-163.
- J. Li, D. Zhang, Q. Que, X. Chen, and K. Ouyang, Ind. Crop. Prod., 2019, 130, 443-449.
CrossRef - S. R. Kareti and P. Subash, Arab. J. Chem., 2020, 13, 6246–6255.
CrossRef - P. Bothi, A. Kaleem, A. Abdul, H. Osman, and K. Awang, Corros. Sci., 2013, 69, 292–301.
CrossRef - G. Ji, S. Anjum, S. Sundaram, and R. Prakash, Corros. Sci., 2015, 90, 107–117.
CrossRef - Y. Feng, J. He, Y. Zhan, J. An, and B. Tan, J. Mol. Liq., 2021, 334, 116110.
CrossRef - H. Fakhry, Colloids Surfaces A Physicochem. Eng. Asp., 2021, 610, 125746.
CrossRef - S. Nikpour, R. Naderi, and M. Mahdavian, J. Taiwan Inst. Chem. Eng., 2018, 88, 261–276.
CrossRef - A. Sehmi, H. B. Ouici, A. Guendouzi, M. Ferhat, O. Benali, and F. Boudjellal, J. Electrochem. Soc., 2020, 167, 155508.
CrossRef - R. Sadeghi Erami, M. Amirnasr, S. Meghdadi, M. Talebian, H.
Farrokhpour, and K. Raeissi, Corros. Sci., 2019, 151, 190–197.
CrossRef - L. M. Mures, R. Bostan, S. Varvara, and L. Ga, Corros. Sci., 2012, 63, 275-286.
CrossRef - S. T. Arab, J. Saudi Chem. Soc., 2011, 15, 73–82.
CrossRef - M. Lebrini, F. Robert, and C. Roos, Int. J. Electrochem. Sci., 2011, 6, 847–859.
CrossRef - B. Tan, J. Ind. Eng. Chem., 2019, 77, 449–460.
- M. Ouakki, Ionics (Kiel)., 2020, 26, 5251–5272.
CrossRef - M. Rbaa, A. S. Abousalem, Z. Roui, R. Benkaddour, P. Dohare, and M. Lakhrissi, surfaces and interfaces, 2020, 19, 100468.
CrossRef - L. L. Liao, S. Mo, H. Q. Luo, and N. B. Li, J. Colloid Interface Sci., 2017.
- L. Li, X. Zhang, J. Lei, J. He, S. Zhang, and F. Pan, Corros. Sci., 2012, 63, 82–90.
CrossRef - T. Yan, J. Taiwan Inst. Chem. Eng., 2020, 106, 118–129.
CrossRef - N. A. Odewunmi, S. A. Umoren, and Z. M. Gasem, Biochem. Pharmacol., 2015, 3, 286–296.
CrossRef - A. Saxena, D. Prasad, and R. Haldhar, Bioelectrochemistry, p., 2018.

This work is licensed under a Creative Commons Attribution 4.0 International License.










