A Review Emphasis on Imbalance of Th1/Th2 Cytokines in The Progression of Diabetes to Diabetic Related Complications
Department of Community Health Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia.
Corresponding Author E-mail: gcmohanphd@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400205
Article Received on : 23 Jan 2024
Article Accepted on :
Article Published : 27 Mar 2024
Reviewed by: Dr. Ioana Stanciu
Second Review by: Dr. Nidhi Sharma
Final Approval by: Dr. Tanay Pramanik
This review describes the imbalance of Th1 and Th2 cytokines during the onset and late complications of diabetes. Systemic inflammation at low-grade is well-known as the basal factor for the onset of diabetes. Several studies have been reported that cytokines have tremendous role in inflammation in type 1 diabetes (T1DM ) as well as type 2 diabetes (T2DM). In general, cellular-mediated immunity is stimulated by Th1 cells whereas Th2 stimulates B cell proliferation and antibody production. It is necessary to understand the role of immune cells secreting chemokines and cytokines, their involvement during the onset and the progression of diabetes particularly diabetic retinopathy/nephropathy. There is clear evidence that type 1 diabetes/autoimmune diabetes is caused by Th1/Th2 cell attacking the beta cells of the pancreas. Numerous cytokines and chemokines contribute to the inflammatory cascade, which may lead to β-cell damage . Th1 cells are the central source of interferon-γ while Th2 cells release interleukins (IL-4, IL-5 and IL-13). Other than diabetes, chronic low-grade inflammation has now stated as a risk factor of various chronic diseases such as obesity, hypertension and dyslipidaemia. In pre-diabetic conditions, the interplay of the pro and anti-inflammatory cytokines has been well understood. However, the association of inflammatory cytokines/chemokines secreted by Th1/Th2 cells in the cause and progression of type 2 diabetes is not fully understood. So, we summarize the results of the blood levels of Th1-Th2 cytokines from the different studies, and whether these cytokine/chemokines can be reported as risk factors for diabetes and their complications such as diabetic retinopathy (DR)/diabetic nephropathy(DN).
KEYWORDS:Diabetes; Diabetic nephropathy; Diabetic retinopathy; T1DM; T2DM; Th1/Th2 cytokines
Download this article as:| Copy the following to cite this article: Govindasamy C. A Review Emphasis on Imbalance of Th1/Th2 Cytokines in The Progression of Diabetes to Diabetic Related Complications. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Govindasamy C. A Review Emphasis on Imbalance of Th1/Th2 Cytokines in The Progression of Diabetes to Diabetic Related Complications. Orient J Chem 2024;40(2). Available from: https://bit.ly/3PCsbop |
Introduction
Diabetes mellitus (DM) is a persistent and enduring health condition. DM disrupts the body’s capacity to effectively utilize the energy derived from food. Diabetes is a group of metabolic disorders which has been associated with hyperglycaemia. Every day, 40-50 units of insulin were secreted by beta (β)-cells of pancreas. Insulin is a polypeptide hormone which help the body cells use glucose for energy. In type 1 diabetes mellitus (T1DM) condition, the pancreas stops producing insulin or produces little insulin. In type 2 diabetes mellitus (T2DM), the pancreas produces insulin, but the insulin can’t bind to its receptor which results in insulin resistance. The global prevalence of DM was estimated to be 9.3% (436 million people) in 2019 which is predicted to grow to 10.2% (578 million people) and 10.9 % (700 million people) by 2030 and 2045, respectively. In urban, the prevalence (10.8%) is found to be higher in comparison to rural (7.2%) areas1. T1DM is formerly recognized as insulin dependent diabetes mellitus (IDDM) which is an autoimmune disease. There is a destruction of its own pancreatic β cell which results in stoppage of production of insulin. It involves 2 or 3 shots of insulin administration with on daily basis. It may occur at any age, and usually appears below age 15. It is highly prone to ketoacidosis with micro or macrovascular complications. It is an idiopathic disease. T2DMwas formerly identified as non-insulin dependent diabetes mellitus (NIDDM) and is associated with obesity. It occurs over age 40 but also occurs in children. Insulin is administered if it is only required. It is more prone to cancer. It is caused by genetic factors 2, 3. Worldwide, around 1 in 11 adults have DM (mostly T2DM) and Asia is the pivotal centre of this widespread T2DM globally4.
Complications of Diabetes
Diabetes increases many risks in health complications like
Microvascular complications (neuropathy and retinopathy)
Macrovascular complications (nephropathy and cardiomyopathy)
Stroke
Diabetic ketoacidosis (DKA) (5)
So, this review covers the results of Th1/Th2 cytokines in T1DM/T2DM and their disease progression has limited to DN/DR.
Diabetic nephropathy
Diabetic nephropathy (DN) is the foremost complication of T2DM worldwide. Poor glycaemic control and fluctuation in blood glucose levels are the major cause of chronic renal disease and cardiovascular death. However, an ethnicity variation of albuminuria in diabetic nephropathy was also observed in Asian, Hispanic and Caucasian population. The incidence of DN in T2DM patients was consistently tends to be high, alarming the clinicians and the scientist to consider this issue by developing approaches for prevention, diagnosis, and treatment of DN. DN has greatly affected the DM patient’s life expectancy. DN is also the key factor of end stage renal diseases in the western countries. The renal risk is similar in patients with T1DM and T2DM6. The association of renal diseases of carbohydrates tolerance was examined in the Pima, an American Indians who have high prevalence of DM. The early phase of DN is a condition considered by structural/functional changes with decreased glomerular filtration rate. Familial aggregation of albuminuria, chronic as well as end stage kidney disease and difference in the prevalence of DN between populations, suggest a strong genetic influence of DN.7
Diabetic Retinopathy
The most common secondary complication of DM is diabetic retinopathy (DR). It has been recognised as micro vascular disease. The prevalence of this disease is approximately 61.2% of males, 88.6% midst 40 and 80 years, nearly two-thirds of the subject were from the south and west regions, and over 50 % had diabetes for above five years. The diagnosis of DR relies on the detection of micro vascular lesions. The key risk factors of DR are duration of the disease, uncontrolled hyperglycaemia, and the presence of dyslipidaemia and hypertension. However, there is a notable discrepancy in risk exists, indicating that other factors such as genetic variations, glycemic variability could contribute to the susceptibility of DR progression8. Further, another concept is that DR is an independent predictive factor of vessels complication of both micro and macrovascular. Thus, the existence of DR should be considered when assessing the cardiovascular diseases (CVD) risk of a diabetes cases. Reactive oxygen species (ROS) is an unstable molecule with impaired electrons that generally contributes in the redox mechanisms of certain factors such as enzymes, protein, and so on (not clear). In normal biological condition, ROS is kept in bay by endogenous antioxidants. Disruption in this equilibrium results in oxidative stress, which serves as an another risk factor implicated in the pathogenesis of DR9. The retina is susceptible tissue that can be easily affected by ROS because of high energy demands and exposure to light. When the equilibrium is wrecked, ROS can cause retinal cell injury by attacking with the other cellular molecules. There are certain changes in terms of physiological and structural changes, arises in several type of cells which includes retinal endothelial cells, neuronal, and retinal pigment epithelium (RPE) prior to clinical symptoms of DR. Early changes such as presence of microaneurysms, WBC adhesion, apoptosis of endothelial cells, pericytes)/loss of pericytes, and neuronal cells. These changes contribute to the breakdown of barrier between inner and outer blood retinal regions in diabetic macular edema, the leading cause of vision damage. Capillary degeneration and formation of acellular capillaries lead to loss of retinal blood flow and consequent hypoxia and retinal neovascularisation, the hallmark of proliferative diabetic retinopathy10.
Th1/Th2 cytokines and pathophysiology of T1DM
There are only few studies that have done to assess the levels of cytokines in the progression of diabetes to diabetes complications such as diabetic retinopathy/nephropathy 11-14. T cells play a key role in the T1DM pathogenesis rather than T2DM. Of course, it is now well understanding that the inflammation and T cell involvement are the underlying mechanism for the onset of diabetes 15. T cells activation plays a key role in the effector cells which is important in the preservation of organs health and survival 16. T-lymphocytes are the key generators of cytokines. T-lympocytes which express CD4 are referred to as T-helper cells, as the primary contributors to cytokine secretion. Th cells can be broadly divided into Th1 and Th2, which are differentiated by the various cytokines which they secrete. But the involvement of T cells in the progression of diabetes to DR/DN are ambiguous. It is well known that the main site of injury/inflammation in type 1 DM is pancreas principally, the Islets of Langerhans. Auto reaction of pancreatic β cells and the cytokines factors in the pathogenesis of T1DM is associated with the derangements in humoral and cellular immunity 16. The previous reports suggest that Th1 cytokines promote the onset while the Th2 cytokines act to protect the onset and development of type 1 DM 17. Interferon gamma(IFN-ϒ) from Th1 cells are known to block the diversity of Th2 cells and their downstream responses whereas Th2 cytokines and IL-10 inhibits the Th1 activation and further affects the secretion of cytokines from Th1 cells. Thus, Th1 and Th2 mutually act each other and maintains the immune homeostasis through their secretions16,18. It was reported that anti-IL-10-mb was unsuccessful in preventing the Th2 autoimmune destruction of islets of Langerhans. Thus, studies have proved that Th2 cytokines particularly IL-10 are the causative factor for the initial stage of type 1 DM, however Th1-cytokines responses are responsible for the stable and persistent attacks on β-cells 19.
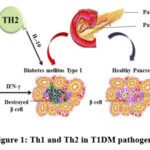 |
Figure 1: Th1 and Th2 in T1DM pathogenesis |
The figure elucidates the role of cytokines in the destruction of Pancreatic beta cells. Th1 cytokine IFN gamma and Th2 cytokine IL-10 are identified as promoters the Beta cell destructions
Th1/Th2 cytokines and pathophysiology of T2DM
It is well established that obesity and the low-grade systemic inflammation are associated with a chief risk of health conditions that including diabetes, hypertension, dyslipidaemia, cancer and Non-alcoholic fatty liver disease 20-23. There is evidence suggest that increase in adiposity reduces plasma glucose efficiency24. Further, there is no link suggested that low grade inflammation reduces the plasma glucose levels. On the other hand, chronic elevation of free fatty acid plays a role in β-cell dysfunction 25, 26. Ceramides, such as sphingosine was found to be elevated in individual with T2 DM. Excess accumulation of ceramides has a link in triggering the inflammatory signalling cascade 27. Tumour Necrosis Factor alpha (TNF-α) signalling are also known to increase ceramide synthesis28. The endocrine function of adipose tissue with respect to insulin sensitizing effects are well established29. The expression and systemic levels of adiponectin are inversely related to insulin resistance 30. Administration of adiponectin in high fructose diet (HFD) mice has been greatly improved tolerance to glucose and sensitivity to insulin31. Unlike T1DM, the principal site of inflammation in T2DM is adipose tissue, the etiology of inflammation is quite different among these two. It was shown that the recruitment of Th1 cells at the early period in adipose tissue32. IFN-ϒ from th1 cells were shown to hinders the insulin signalling cascade and insulin-induced glucose uptake in T2DM 33.
In T2DM, the level of th2 cytokines (IL-4, IL-5 and IL-13) is significantly increased (34). T2DM has low-grade inflammation at both the systemic and organ level as well. But still the game played by th1 and th2 cytokines are in paradox. The role played by inflammation in T2DM is complex and multifactorial. IL-6 is the well characterized proinflammatory cytokine. They are known to involved in the progression of T2DM to DR. IL-6 is the only cytokines had gradually increased among the various cytokines studied11 . So, IL-6 was the vital cytokine related to pathogenesis of Type 2 DM to its complications. T cell cytokine play a significant role in β-cells of pancreas/pancreatic function 35. Besides, IL-6 signalling in liver is known to stimulate the levels of acute phase response by increasing C-reactive protein and serum amyloid proteins36. Systemic levels of tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein (MIP-1α), IL-1 and IL-8 are also elevated in the onset of T2DM37. However, the involvement of Th1/Th2 cytokines with the other non-communicable disease such as cancer is well established. They are participating in the tumour immune biology. Th1 can be good prognostic marker especially in patients with hepatic carcinoma whereas Th2 are known to progressive in tumour metastasis 38.
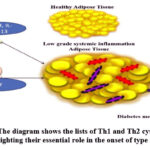 |
Figure 2: The diagram shows the lists of Th1 and Th2 cytokines and highlighting their essential role in the onset of type 2-DM |
Focus on Th1/Th2 cytokines towards the etiology of Diabetes complications
Over the past few decades, T2DM pathology and its progression to DN/DR are associated with metabolic abnormalities and hemodynamic factors other than inflammation. Inflammation is the major factor seems to triggers the severity of the disease. But still T cells are profoundly altered and subsequently the cytokines have consistent role in T1DM to DR/DN progression. Only limited information is available for the role played by Th1 and Th2 in the onset and progress of diabetes complications under T2DM condition. There is a lot of avenues to explore the involvement of cytokines in the progression of T2DM to DR/DN. Imbalance in the Th1/Th2 profile may lead to illness. Studies have also been suggested that DM is associated with immune system and the cytokines are involved in the disease progression 39. Leukocyte infiltration and the sluggish blood flow in the retinal blood vessels triggers the pathophysiology of DR that include which including basement thickening, blood retinal barrier (BRB) leakage, apoptosis and the detachment of retina40. Further, the interplay of inflammatory factors such as cytokines, chemokines and angiogenic factors are responsible for the disease progression of DR. It has been reported that both mRNA and protein level of Th1 cytokines (IL-2 and TNF-α) are significantly higher in DR when compared to T2DM41. Unlike mRNA, the protein level of Th2 cytokines was lower in DR, suggesting that Th1/Th2 imbalance is intricated with the occurrence as well as DR development41. Th1/Th2 cells maintain the homeostasis by altering the release of cytokines, that plays a significant role in cellular and humoral immunity. These results further suggest that Th-1 cytokines triggers an inflammatory response by promoting leukocyte infiltration and the migration of immune cells. Inflammatory response causes the damage of retinal ganglion cells which may leads to occurrence and progression of DR 42. Studies also says that high blood levels of IL-10 have negative impact in the prevalence of DR43. It has been reported that serum and vitreous of IL-6 and IL-10 are altered in proliferative diabetic retinopathy (PDR) 43. A study on profiling of cytokine has revealed that there were no any significant changes observed between the DM with/without DR though the disease duration of DR is higher44. Masaru et al stated that TNF-α, IL-6 and IL-4 level in vitreous are higher as compared to serum 43, thus it remains unclear that Th1 and Th2 cytokines would have a facilitate/inhibitory role in the development of DR. This study also stated that Th2 and Th17 related inflammatory response rather than Th1 may involve in the pathogenesis of DR 43.
Glomerular hypertrophy, basement membrane thickening and deposition of extracellular components can be of primary changes associated with the pathogenesis of DN 45,46. In earlier days, hemodynamic parameters (blood pressure, and total cardiac output peripheral resistance) and metabolic syndrome were considered the primary causes of renal injury in patients with T2DM and DN which were believed to be non-inflammatory disease 47. Beside, augmented infiltration of macrophages and activated T lymphocytes, along with the increased levels of pro inflammatory cytokines in the renal lesions have been identified in DN cases 48,49. Previous studies have shown the evidence that the culturing of peritoneal macrophages with glomerular basement membrane of diabetic kidneys secretes significant quantities of TNF-α and IL-1 as compared with the glomerular basement membrane of normal kidneys 50. Research indicates that imbalances of Th1/Th1 cells are involved in the pathogenesis of T1DM, however the involvement of T cells with the progression of DN are still to be expound more. It is well known that Th1 cells are more dominant in T1DM; so, it is perhaps that T-cells is primarily involved in the disease progression of T1DM to DN. It has been found that increased levels of IFN-ϒ (Th1 cells) show positive correlation with proteinuria and GFR of DN patients 51. Further, an increased IL-2 levels positively correlated with proteinuria of DN patients51. The Th2 cytokines such as IL-4 and IL-10 secretion was reduced in DR as compared to DM without DR, Th1 cytokines as described above are found negatively correlated with IL-4 and IL-10 42. In contrast to DN immune pathology, there was no any significant changes of serum IL-4 in DN patients in comparison to DM but without DN. IL-10, was reduced in significant manner in DN patients compared to DM suggesting their immunosuppressive and anti-inflammatory role 52, 53. Studies have also reported that serum IL-10 is significantly increased in DN patients which is positively correlated with albuminuria 54. It has been reported that TNF-α, IL-6, IL-1, IL- 18 are the major candidates, performing various activities which is involved in the progression of DN. A study on profiling of cytokines on DN has shown that the levels of IL-12, IL-14 and IFN-ϒ are increased whereas IL-33 level was decreased 55. Numerous studies have clearly shown that the imbalance of Th1/Th1 cytokines not only occur at the level of onset of DM. It may have persistant role in the immune pathology progression of DM to DR/DN (Fig.3).
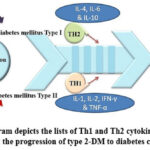 |
Figure 3: The diagram depicts the lists of Th1 and Th2 cytokines and highlighting their role in the progression of type 2-DM to diabetes complications |
Immune pathogenesis underlying type 2 diabetes, with a focus on the role of Th1/Th2 and the other subtypes are remains at an early stage. Though, studies have identified cytokines which are involved in the onset and the progression of type 2 DM to DR/DN, but further studies are warranted to explore mechanism for preventing onset/progression of the disease. Moreover, investigations such as wet lab/molecular docking simulation are required to explore the involvement of Th1 and Th2 cytokines and their interactions to other biological molecules in the disease progression. Ultimately, it is important to develop the pathway/strategies aimed at preventing onset/disease progression and prolonging the lifespan of the patients with diabetes.
Conclusion
Understanding the imbalance of Th1/Th2 immune biology in the context of DM to DN/DR will provide a better clinical outcome in the treatment of DM complications. The involvement of T-cells in DM progression and the comparison of T-cell immune response between T1DM and T2DM is yet to be elucidated. Cells of immune system and various cytokines play a vital role in complex pathology of DN/DR. Deep understanding of immune function of Th1/Th2 cells can be a key for new prognostic marker development. Also, it is necessary for therapeutic strategies development such as either adjuvant immune therapy or monotherapy in addition to existing treatment which have greater vision in managing the DM and its disease progression.
References
- American Diabetes Association. Diabetes care., 2019, S13-S28.
- Olokoba, AB.; Obateru, OA.; Olokoba, LB.; T. Oman medical journal., 2012, 27(4), 269-73.
CrossRef - Pradeepa, R.; Mohan, V. Indian journal of ophthalmology., 2021, 69(11),2932-8.
CrossRef - Kahanovitz, L.; Sluss, PM.; Russell, SJ. A Clinical Perspective Point of care., 2017, 6(1), 37-40.
CrossRef - Chawla, A.; Chawla, R.; Jaggi, S. Indian journal of endocrinology and metabolism., 2016, 20(4), 546-51.
CrossRef - Kos, I.; Prkacin, I. Acta medica Croatica., 2014, 68(4-5), 375-81.
- Persson, F.; Rossing, P. Kidney international supplements. 2018, 8(1), 2-7.
CrossRef - Duh, EJ.; Sun, JK,; Stitt, AW. JCI insight. 2017, 20(2) 14.
CrossRef - Pickering, RJ.; Rosado, CJ.; Sharma, A.; Buksh, S.; Tate, M.; de Haan, JB. Clin Transl Immunology.,.2018, 7(4), 1016.
CrossRef - Kowluru, RA.; Chan, PS. Experimental diabetes research., 2007, 43603.
CrossRef - Kaviarasan, K.; Jithu, M.; Arif Mulla, M.; Sharma ,T.; Sivasanka,r S.; Das, UN. Metabolism: clinical and experimental., 2015, 64(9), 958-66.
CrossRef - Zhao, Z. Journal of musculoskeletal & neuronal interactions., 2018, 18(3), 348-53.
- Sifnaios, E.; Mastorakos, G.; Psarra, K.; Panagopoulos, ND.; Panoulis, K.; Vitoratos, N. In vivo. 2019, 33(1), 1-40.
CrossRef - Wu, CC.; Sytwu, HK.; Lu KC.; Lin, Y. Experimental diabetes research., 2011, 514738.
- Oguntibeju, OO. International journal of physiology, pathophysiology and pharmacology. 2019, 11(3),45-63.
- Almawi, WY.; Tamim, H.; Aza,r ST. The Journal of clinical endocrinology and metabolism., 1999, 84(5),1497-502.
CrossRef - Manetti, R.; Parronchi, P.; Giudizi, MG.; Piccinni, MP.; Maggi, E.; Trinchieri, G. The Journal of experimental medicine., 1993, 1;177(4),1199-204.
CrossRef - Romagnani, S. Journal of clinical immunology., 1995, 15(3),121-9.
CrossRef - Pakala, SV.; Kurrer, MO.; Katz, JD. The Journal of experimental medicine., 1997, 186(2),299-306.
CrossRef - Adams, LA.; Angulo, P.; Lindor, KD. Canadian Medical Association journal., 2005 172(7),899-905.
CrossRef - Duncan, BB.; Schmidt, MI.; Pankow, JS.; Ballantyn,e CM.; Couper, D.; Vigo, A. Diabetes., 2003, 52(7),1799-805.
CrossRef - Renna, NF. International journal of hypertension., 2013;710136.
CrossRef - Greten, FR.; Grivennikov, SI. Immunity., 2019, 16-51(1), 27-41.
CrossRef - Burhans, MS.; Hagman, DK.; Kuzma, JN.; Schmidtm KA.; Kratz, M. Comprehensive Physiology., 2018, 13;9(1), 1-58.
CrossRef - Meikle, PJ. Summers, SA.; Nature reviews Endocrinology., 2017, 13(2), 79-91.
CrossRef - Boden, G. Laakso, M.; Diabetes care., 2004, 27(9), 2253-9.
CrossRef - Haus, JM.; Kashyap, SR.; Kasumov, T.; Zhang, R.; Kelly, KR.; Defronzo, RA. Diabetes., 2009., 8(2), 337-43.
CrossRef - Hernandez-Corbacho, MJ.; Canals, D.; Adada, MM.; Liu, M.; Senkal, CE.; Yi, JK. The Journal of biological chemistry., 2015, 16;290(42), 25356-73.
CrossRef - Eglit, T.; Ringmets, I.; Lembe,r M. PloS one., 2013., 8(9), e73273.
CrossRef - Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K. Nature medicine., 2001, 7(8),941-6.
CrossRef - Sharma, NK.; Da,s SK.; Mondal, AK.; Hackney, OG.; Chu, WS.; Kern, PA. The Journal of clinical endocrinology and metabolism., 2008, 93(11),4532-41.
CrossRef - Anderson, JT.; Cornelius, JG.; Jarpe, AJ.; Winter, WE.; Peckm AB. Autoimmunity., 15(2),113-22.
CrossRef - Stalenhoef, JE.; Alisjahbana, B.; Nelwan, EJ.; van der Ven-Jongekrijg, J.; Ottenhoff, TH/; van der Meer, JW. European Society of Clinical Microbiology., 2008, 27(2),97-103.
CrossRef - Surendar, J.; Mohan, V.; Rao, MM.; Babu, S.; Aravindhan, V. Diabetes technology & therapeutics., 2011, 13(4),477-82.
CrossRef - Wang, C.; Guan, Y.; Yang, J. International journal of endocrinology., 2010., 515136.
CrossRef - Castell, JV.; Gomez-Lechon, MJ.; David, M.; Andus, T.; Geiger, T.; Trullenque, R. FEBS letters., 1989, 2;242(2), 237-9.
CrossRef - Goyal, R;, Faizy, AF.; Siddiqui, SS.; Singhai, M. North American journal of medical sciences., 2012, 4(4),180-4.
CrossRef - Ma, L.; Zeng, J.; Mo, B.; Wang, C.; Sun, Y. Zhang, M. International journal of clinical and experimental medicine., 2015, 8(4),5121-8.
CrossRef - Wilkinson-Berka, JL.; Wraight, C.; Werther, G. Current medicinal chemistry. 2006;13(27):3307-17. PubMed PMID: 17168853.
- Cao, YL.; Zhang, FQ.; Hao, FQ. Genetics and molecular research ., 2016, 15(3).
CrossRef - Hao, M.; Li, Y.; Lin, W.; Xu, Q.; Shao, N,.; Zhang, Y. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie., 2015, 253(1):83-90.
- Takeuchi, M.; Sato, T.; Tanaka, A.; Muraoka, T.; Taguchi, M.; Sakurai, Y. PloS one., 2015, 10(9), e0137358.
- Lee, JH.; Lee, W.; Kwon, OH.; Kim, JH.; Kwon, OW.; Kim, KH. Annals of clinical and laboratory science., 2008, 38(4):361-7.
CrossRef - Zeisberg, M.; Neilsonm EG. Journal of the American Society of Nephrology., 2010 21(11),1819-34.
- Hoshino, J.; Mise, K.; Ueno, T. Imafuku, A.; Kawada, M.; Sumida, K.; American journal of nephrology., 2015, 41(4-5), 337-44.
CrossRef - Zheng, Z.; Zheng, F. Journal of diabetes research., 2016, 1841690.
CrossRef - Festa, A.; D’Agostino, R.; Howard, G.; Mykkanen, L.; Tracy, RP.; Haffner, SM. Circulation., 2000,4;102(1), 42-7.
CrossRef - Frohlich, M.; Imhof, A.; Berg, G.; Hutchinson, WL.; Pepys, MB.; Boeing, H. Diabetes care., 2000, 23(12), 1835-9.
CrossRef - Harjutsalo, V.; Groop, PH. 2014, 21(3), 260-6.
CrossRef - Wu, CC.; Chen, JS.; Lu, KC.; Chen, CC.; Lin, SH.; Chu P. Clinica chimica acta; international journal of clinical chemistry., 2010, 411(9-10), 700-4.
CrossRef - Lalani, I.; Bhol, K.; Ahmed, AR. Annals of allergy, asthma & immunology., 1997, 79(6),469-83.
CrossRef - Van Exel, E.; Gussekloo, J.; de Craen AJ.; Frolich, M.; Bootsma-Van Der Wiel, A.; Westendorp, RG. Diabetes., 2002, 51(4),1088-92.
CrossRef - Mysliwska, J.; Zorena, K.; Semetkowska-Jurkiewicz E.; Rachon, D.; Suchanek, H.; Mysliwski, A. European cytokine network., 2005, 16(2),117-22.
CrossRef - Anand, G.; Vasanthakumar, R.; Mohan, V.; Babu, S.; Aravindhan, V. International journal of clinical and experimental pathology., 2014, 7(11), 8008-15.
Abbreviation
Diabetes Mellitus : DM
Type 2 DM : T2DM
Type 1 DM : T1DM
Insulin dependent diabetes mellitus : IDDM
Non-Insulin dependent diabetes mellitus : NIDDM
Diabetic keto acidosis : DKA
Diabetic Retinopathy : DR
Diabetic Nephropathy : DN
Reactive Oxygen Species : ROS
End Stage Renal Disease : ESRD
Interferon -ϒ : IFN-ϒ

This work is licensed under a Creative Commons Attribution 4.0 International License.










