Synthesis of Novel Indolylbenzothiazepines/Indolylbenzoxaziepines Substituted 2-Oxo/Thiobarbituric Acids as Potential Anticonvulsant Agents.
1Medicinal Chemistry Laboratory, Department of Chemistry, Meerut College, Meerut, (U.P.) India.
2Department of Chemistry, D.N.P.G. College, Meerut, (U.P.) India.
Corresponding Author E-mail: archanachemistrymcm@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400129
Article Received on : 06 Sep 2023
Article Accepted on :
Article Published : 09 Jan 2024
Reviewed by: Dr. Netra Pal Singh
Second Review by: Dr. Anita Sharma
Final Approval by: Dr. Nenad Ignjatovic
4-(2’-Oxo/thiobarbiturinyl acid) – 2 - (2”-halo-1”H-indolyl) - 2,3 - dihydro - -1,5-benzothiazepines (7-10) and 4-(2’-oxo/thiobarbiturinyl acid) – 2 - (2”-halo-1”H-indolyl) - 2,3 - dihydro - -1,5-benzoxazepines (11-14) undergoes Mannich reaction to afford compounds 4-(2’-oxo/thiobarbiturinyl acid) – 2 - (2”-halo-1”H-indolyl) – 3 - (substitutedphenyl aminomethylene) - 2,3 - dihydro - 1,5-benzothiazepines (15-22) and 4-(2’-oxo/thiobarbiturinyl acid) – 2 - (2”-halo-1”H-indolyl) – 3-( substitutedphenyl aminomethylene) - 2,3 - dihydro - 1,5-benzoxazepines (23-30) correspondingly. All the chemical framework of these newer drugs were elucidated by using elemental and IR and NMR spectroscopy. All these newly synthesized compounds were tested for antiepileptic effect against SMES experimental models and the results were collated with phenytoin sodium - standard drug. Results of antiepileptic profile showed promising effect in most of the derivatives synthesized. Activity equal to standard drug was shown by compounds 9 and 28. The most promising and active compound of this project was found to be 4-(2’- thiobarbiturinyl acid) – 2 - (2”-chloro-1”H-indolyl) – 3 - (chlorophenyl aminomethylene) - 2,3 - dihydro - 1,5-benzothiazepines, which elicited activity greater than the standard drug. All the antiepileptic drugs of the produced in this projects were also tested for ALD50.
KEYWORDS:Antiepileptic activity; ALD50; Benzothiazepines, Benzoxazepines, Indoles, oxo/thiobarbituric acid, Synthesis
Download this article as:| Copy the following to cite this article: Archana A, Awasthi A, Chaudhary S. Synthesis of Novel Indolylbenzothiazepines/Indolylbenzoxaziepines Substituted 2-Oxo/Thiobarbituric Acids as Potential Anticonvulsant Agents. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Archana A, Awasthi A, Chaudhary S. Synthesis of Novel Indolylbenzothiazepines/Indolylbenzoxaziepines Substituted 2-Oxo/Thiobarbituric Acids as Potential Anticonvulsant Agents. Orient J Chem 2024;40(1). Available from: https://bit.ly/3tRQ1EP |
Introduction
Epilepsy is a disorder related to the nervous system affecting the brain and its activity by causing frequent seizures and sometimes, loss of awareness. In simple words, epilepsy is a disorder or disease of the brain with a potential of making a human unconscious. A group of medicative agents that are used for the treatment of epilepsy are referred to as anticonvulsant agents. Although a large number of anticonvulsant drugs are present and are being used for the treatment of epileptic seizures, yet their long exposure can lead to drug resistance. In addition, the presently available drugs are also associated with severe side effects such as hypnosis, sedation, etc. thereby retarding everyday performance. As per epidemiological studies 1-3, currently the disease affects more than 60 million people worldwide. Thus, a novel and a safer anticonvulsant drug is the most needed scientific discovery of today.
Mephobarbital 4 and phenobarbital 5, which are derivatives of 2,4,6-tri-oxo-hexahydropyrimidine(barbituric acid) are medically being used for the treatment of epilepsy. The substitution with various alkyl, aryl or heteroaryl moieties at fifth position of barbituric acid 6-9 and thiobarbituric acid 9-12 was found to plays an essential role in modulating the antiepileptic effect. Indoles 13-17 have been found to possess antiepileptic activity in maximal electroshock seizure model experiment. Literature survey reveals that derivatives of benzo-thiazepines as well as benzo-oxazepines were used in the synthesis and design of new and different antiepileptic drugs. The chemistry and pharmacology of benzothiazepine and benzoxazepine derivatives have been of substantial attraction to health professionals for the reason that their by-products were found to possess various biotic activities such as antiinflammatory 18, antimicrobial 18-19, antioxidant 20, antitumor 21, antipsychotic 22-23, anticonvulsant 23-26, etc. In view of above discussion the motive of this study is to synthesize newer antiepileptic drugs by embodying benzothiazepine and benzoxazepine segments on 2-oxo / thiobarbituric acids (at fifth position) which was already incorporated with indole moiety. The introduction of these components on 2-oxo/thiobarbituric acids (at fifth position), may be innovative as the substituted groups themselves possess anticonvulsant effect and their introduction on 2-oxo / thiobarbituric acids (at fifth position) was further expected to enhance the antiepileptic activity.
Material and Methods
Chemistry
Melting points were recorded with the help of thermonic melting point apparatus. The open capillary tubes were used for taking the melting points which were faulty (not correct). The homogeneousness and pureness of the drugs produced were examined using TLC (thin layer chromatography) utilizing plates of silica gel-G. Methanol-benzene mixture in the ratio of 2:8 was used as an eluent. The spots of TLC were located with the help of iodine. Carlo Erba 1108 analyzer was used to perform elemental analysis (C, H, N) and were found within the range of ± 0.04 % of the conceptual values. Perkin Elmer 881 FT-IR spectrophotometer ( νmax in cm-1 ) was used to record IR spectra. Bruker DRX-300-FTNMR instrument was used to record 1H-NMR spectra in CDCl3 .
Compounds (1-14) were synthesized according to the routes depicted in Figure-1. The physical data (melting points, recrystallization solvents, yields, molecular weights, elemental analysis) of derivatives (1-14) is stated in Table-1.These synthesized drugs (1-14) were also evaluated for their antiepileptic activeness and also for their ALD50 ( acute toxicity). The standard drug used for antiepileptic activity was phenytoin sodium. The results of biological activity that is antiepileptic acitivty and acute toxicity is depicted in (Table-2).
Table 1: Physical and analytical details of compounds 1-30
|
Comp. No. |
X |
R |
R’ |
M.P. (0C) |
Recryst. Solvent |
Yield (%) |
Molecular Formula |
Calcd. (Found) % |
||
|
C |
H |
N |
||||||||
|
1. |
O |
– |
– |
180 |
methanol |
46 |
C6H6O4N2 |
42.35 (42.30) |
3.52 (3.50) |
16.47 (16.50) |
|
2. |
S |
– |
– |
250 |
ethanol |
66 |
C6H6O3N2S |
38.70 (38.68) |
3.22 (3.19) |
15.05 (15.08) |
|
3. |
O |
Cl |
– |
240 |
DMF |
62 |
C15H10O4N2Cl |
56.69 (56.72) |
3.14 (3.17) |
8.81 (8.79) |
|
4. |
O |
Br |
– |
280 |
acetone |
55 |
C15H10O4N2Br |
49.72 (49.68) |
2.76 (2.78) |
7.73 (7.69) |
|
5. |
S |
Cl |
– |
275 |
ethanol |
58 |
C15H10O3N2 ClS |
53.97 (54.00) |
2.99 (3.01) |
8.39 (8.42) |
|
6. |
S |
Br |
– |
250 |
methanol |
48 |
C15H10O3N2BrS |
47.61 (47.57) |
2.64 (2.67) |
7.40 (7.36) |
|
7. |
O |
Cl |
– |
150 |
ethanol |
42 |
C21H16O3N4 ClS |
57.33 (57.35) |
3.64 (3.66) |
12.74 (12.77) |
|
8. |
O |
Br |
– |
230 |
pet.ether |
55 |
C21H16O3N4BrS |
52.06 (52.09) |
3.30 (3.27) |
11.57 (11.61) |
|
9. |
S |
Cl |
– |
210 |
ethanol/ water |
44 |
C21H16O2N4ClS2 |
55.32 (55.29) |
3.51 (3.48) |
12.29 (12.32) |
|
10. |
S |
Br |
– |
200 |
toluene |
46 |
C21H16O2N4BrS2 |
50.40 (50.37) |
3.20 (3.17) |
11.20 (11.18) |
|
11. |
O |
Cl |
– |
225 |
acetone |
42 |
C21H16O4N4Cl |
59.50 (59.59) |
3.77 (3.80) |
13.22 (13.19) |
|
12. |
O |
Br |
– |
260 |
ethanol |
40 |
C21H16O4N4Br |
53.84 (53.86) |
3.41 (3.39) |
11.96 (12.00) |
|
13. |
S |
Cl |
– |
235 |
DMF |
58 |
C21H16O3N4ClS |
57.33 (57.36) |
3.64 (3.67) |
12.74 (12.71) |
|
14. |
S |
Br |
– |
225 |
methanol |
48 |
C21H16O3N4BrS |
52.06 (52.09) |
3.30 (3.26) |
11.57 (11.69) |
|
15. |
O |
Cl |
4-OCH3 |
180 |
ethanol |
46 |
C29H25O4N5ClS |
60.57 (60.60) |
4.35 (4.38) |
12.18 (12.21) |
|
16. |
O |
Cl |
4-Cl |
160 |
methanol |
40 |
C28H22O3N5Cl2S |
58.13 (58.09) |
3.80 (3.77) |
12.11 (12.08) |
|
17. |
O |
Br |
4-OCH3 |
155 |
ethanol |
62 |
C29H25O4N5BrS |
56.21 (56.18) |
4.03 (4.06) |
11.30 (11.27) |
|
18. |
O |
Br |
4-Cl |
225 |
ethanol |
40 |
C28H22O3N5BrClS |
53.88 (53.91) |
3.52 (3.49) |
11.22 (11.19) |
|
19. |
S |
Cl |
4-OCH3 |
200 |
DMF |
46 |
C29H25O3N5ClS2 |
58.98 (59.00) |
4.23 (4.19) |
11.86 (11.89) |
|
20. |
S |
Cl |
4-Cl |
230 |
methanol |
38 |
C28H22O2N5Cl2S2 |
56.56 (56.52) |
3.70 (3.67) |
11.78 (11.80) |
|
21. |
S |
Br |
4-OCH3 |
205 |
acetone |
42 |
C29H25O3N5BrS2 |
54.80 (54.77) |
3.93 (3.89) |
11.02 (11.05) |
|
22. |
S |
Br |
4-Cl |
245 |
methanol |
40 |
C28H22O2N5BrClS2 |
52.54 (52.57) |
3.44 (3.47) |
10.94 (10.97) |
|
23. |
O |
Cl |
4-OCH3 |
260 |
pet.ether |
36 |
C29H25O5N5Cl |
62.30 (62.27) |
4.47 (4.50) |
12.53 (12.49) |
|
24. |
O |
Cl |
4-Cl |
240 |
ethanol |
46 |
C28H22O4N5Cl2 |
59.78 (59.91) |
3.91 (3.89) |
12.45 (12.48) |
|
25. |
O |
Br |
4-OCH3 |
235 |
ethanol |
48 |
C29H25O5N5Br |
57.71 (57.68) |
4.14 (4.17) |
11.60 (11.57) |
|
26. |
O |
Br |
4-Cl |
195 |
ethanol |
50 |
C28H22O4N5BrCl |
55.30 (55.27) |
3.62 (3.59) |
11.52 (11.49) |
|
27. |
S |
Cl |
4-OCH3 |
215 |
ethanol |
45 |
C29H25O3N5ClS |
62.30 (62.27) |
4.47 (4.50) |
12.53 (12.50) |
|
28. |
S |
Cl |
4-Cl |
205 |
acetone |
42 |
C28H22O3N5Cl2S |
58.13 (58.15) |
3.80 (3.78) |
12.11 (12.09) |
|
29. |
S |
Br |
4-OCH3 |
250 |
methanol |
44 |
C29H25O4N5BrS |
56.21 (56.18) |
4.03 (4.00) |
11.30 (11.26) |
|
30 |
S |
Br |
4-Cl |
200 |
methanol |
44 |
C28H22O3N5BrClS |
53.88 (53.91) |
3.52 (3.49) |
11.22 (11.19) |
C, H, N were found within ±0.04%
Table 2: Pharmacological details of compounds (1-30).
|
Compound Number |
Acute Toxicity ALD50 (mg/kg i.p.) |
Anticonvulsant Activity |
|
|
Dose (mg/kg i.p.) |
Percentage Inhibition of Seizures |
||
|
1. |
>1000 |
30 |
40* |
|
2. |
>1000 |
30 |
40* |
|
3. |
>1000 |
30 |
50* |
|
4. |
>1000 |
30 |
40* |
|
5. |
>1000 |
30 |
60** |
|
6. |
>1000 |
30 |
50** |
|
7. |
>1000 |
30 |
60* |
|
8. |
>1000 |
30 |
50** |
|
9. |
>1000 |
7.5 15 30 |
30* 60** 80*** |
|
10. |
>1000 |
30 |
60** |
|
11. |
>1000 |
30 |
50* |
|
12. |
>1000 |
30 |
50** |
|
13. |
>1000 |
30 |
70** |
|
14. |
>1000 |
30 |
60** |
|
15. |
>1000 |
30 |
70*** |
|
16. |
>1000 |
30 |
80** |
|
17. |
>1000 |
30 |
60** |
|
18. |
>1000 |
30 |
70*** |
|
19. |
>1000 |
30 |
70** |
|
20. |
>1000 |
7.5 15 30 |
50* 60** 90*** |
|
21. |
>1000 |
30 |
60** |
|
22. |
>1000 |
30 |
70* |
|
23. |
>1000 |
30 |
70** |
|
24. |
>1000 |
30 |
70** |
|
25. |
>1000 |
30 |
60* |
|
26. |
>1000 |
30 |
70** |
|
27. |
>1000 |
30 |
70** |
|
28. |
>1000 |
7.5 15 30 |
40** 60** 80*** |
|
29. |
>1000 |
30 |
70** |
|
30. |
>1000 |
30 |
70*** |
|
Phenytoin Sodium |
|
30 |
80*** |
|
Propylene Glycol |
|
2.0 |
0 |
*p<0.05, **p<0.01, ***p<0.001
Synthesis
General procedure to synthesize 5-methoxy-2- thio/ oxo barbituric acids (1-2)
To 2-thio/oxobarbituric acid (20 g), acetyl chloride (50 ml) was add on little by little with regularly moving the liquid round and round and maintaining the temperature between 0-50 C. Magnetic stirrer was used to stir the reaction mixture for another 10 hours. After stirring the mixture was kept overnight. Distillation assembly was used to distill off excess of acetic acid. The solid residue obtained after distillation was washed with water again and again and was then poured into ice. Filtration pump was used to filter the solid residue. The solid residue thus obtained was filtered which was again solidified using suitable solvents. The analytical as well as physical details of compounds 1-2 is stated in Table 1. Compound 1: 1H-NMR CDCl3 δ: 5.80 (s, 1H, CHCOCH3), 2.55 (s, 3H, COCH3), 9.20 (s, 2H, 2N-HC=O). IR (cm-1, KBr): 1740, 1720, 1690, 1700 (C=O), 3180 (-N-H).
General procedure to synthesize 1-(2’-oxo/thiobarbiturinyl acid)-3-(2”-halo-1”H-indolyl)-prop-2-en-1-ones (3-6)
Compounds 1-2 (0.01 mole) were fused in pure ethanol (50 ml). To this mixture obtained, 3-formyl-2-halo-1H-indoles (0.01 mol) were put on. Refluxing of reaction mixture was done for about 12 hours in the presence of 60% KOH. These reaction mixtures obtained after refluxing were evaporated, chilled and finally gushed onto ice. Filtration pump was used to produce solids. The solids produced were filtered. The solids obtained after filtration were further cleaned with petroleum ether (40-600 C), after which they were crystallized again using suitable solvents. The analytical as well as physical details of compounds 3-6 are specified in Table 1. Compound 3: 1H-NMR CDCl3 δ: 8.99 (s, 2H, 2NHCO), 9.80 (brs, 1H, N-H of indole), 5.65 (m, 1H, CHCO), 6.95 – 7.25 (m, 4H, Ar-H), 8.45 (d, 1H, =CH-), 6.55 (d, 1H, -COCH=),. IR (cm-1, KBr): 3180 (N-H of indole), 1585 (C-C of aromatic ring), 1750, 1710, 1700,1730 (C=O), 710 (C-Cl), 1625 (CH=CH).
General procedure to synthesize 4-(2’-oxo/thiobarbiturinyl acid) – 2 – (2”-halo-1”H-indolyl) – 2,3 – dihydro – -1,5-benzo-thiazepines (7-10)
1-(2’-Oxo / thiobarbiturinyl acid)-3-(2”-halo-1”H-indolyl)-prop-2-en-1-ones (3-6) (0.01 mole) were added to methanol (50 ml) to form various solutions. 2-amino-thiophenol (0.01 mole) having some drops of extremely cold acetic acid (1 ml) was also added to the solutions formed. These solutions were refluxed for 4-5 hours. The execution of reactions were noticed by TLC. Then after, extra solvents were distilled off using distillation assembly under lesser pressure to get solid remnant. The solids which were produced after distillation were resolidified by using appropriate solvents to afford compounds 7-10. The analytical as well as physical details of compounds 7-10 are stated in Table 1. Compound 7: 1H-NMR CDCl3 δ: 5.60 (m, 1H, CH of barbituric acid), 9.12 (ss, 2H, 2NHCO), 9.88 (brs, 1H, N-H of indole), 6.90-7.68 (m, 8H, Ar-H), 6.59 (s, 1H, C2-Hof thiazepine ring), 7.25 (d, 2H, C3-H of thiazepine ring), 4.25 (t, 1H, C4-H of thiazepine ring). IR (cm-1, KBr): 3188 (N-H of indole), 1740, 1710, 1720 (C=O), 717 (C-Cl), 1616 (C=C), 690 (C-S-C), 3045 (aromatic C-H), 1585 (C-C of aromatic ring), 690 (C-S-C), 1485 (C-N).
General procedure to synthesize 4-(2’-oxo/thiobarbiturinyl acid) – 2 – (2”-halo-1”H-indolyl) – 2,3 – dihydro – -1,5-benzoxazepines (11-14)
Methanol about 50 ml and 1-(2’-Oxo/thiobarbiturinyl acid)-3-(2”-halo-1”H-indolyl)-prop-2-en-1-ones (3-6) (0.01 mol) were mixed together and a clear solution was formed. 2-Aminophenol containing some drops of glacial acetic acid (1 ml) were put in together to this methanolic solutions of compounds (3-6) and were refluxed for about 4-5 h. The progression of the reactions were determined by TLC. The extra solvents were distilled off using distillation assembly with the reduced pressure. The solid yields which were produced were crystallized again by using suitable and appropriate solvents to yield compounds 11-14. The analytical as well as physical details of compounds (11-14) are mentioned in Table 1. Compound 14: 1H-NMR CDCl3 δ: 5.66 (m, 1H, CH of barbituric acid), 9.15 (ss, 2H, 2NHCO), 7.10-7.88 (m, 8H, Ar-H), 6.50 (s, 1H, C2-Hof oxazepine ring), 9.85 (brs, 1H, NH of indole),7.68 (d, 2H, C3-H of oxazepine ring), 4.20 (t, 1H, C4-H of oxazepine ring). IR (cm-1, KBr): 3040 (aromatic CH), 3192 (NH of indole), 1735, 1720, 1715 (C=O), 720 (C-Cl), 1620 (C=C), 1488 (C-N), 1588 (C-C of aromatic ring), 1666 (C=N), 1070 (C-O-C).
General procedure to synthesize 4-(2’-oxo/thiobarbiturinyl acid) – 2 – (2”-halo-1”H-indolyl) – 3 – (substitutedphenyl aminomethylene) – 2,3 – dihydro – 1,5-benzothiazepines (15-22)
4-(2’-Oxo / thiobarbiturinyl acid) – 2 – (2”-halo-1”H-indolyl) – 2,3 – dihydro – 1,5-benzothiazepines (7-10) (0.001 mole), formaldehyde (0.01 mole) and anilines having different substitutions (0.001 mole) in methanol (50 ml) are mixed together to form clear solutions. The solutions formed were refluxed for nearly 5-6 hours. On accomplishment of refluxing, the mixtures produced were first evaporated, then chilled and finally put onto ice. After doing so semi-solid products were separated. The semi solids which separated out were kept in petroleum ether (40-600 C) and were left ovenight. The next day solid products were produced, which were crystallized again with suitable solvents. The analytical as well as physical details of the compounds (15-22) are stated in Table-1. Compound 15: 1H-NMR CDCl3 δ: 5.60 (m, 1H, CH of barbituric acid), 9.12 (ss, 2H, 2NHCO), 9.88 (brs, 1H, N-H of indole), 7.68-6.80 (m, 12H, Ar-H), 3.45 (s, 3H, OCH3),6.59 (s, 1H, C2-Hof thiazepine ring), 7.25 (d, 2H, C3-H of thiazepine ring), 4.25 (t, 1H, C4-H of thiazepine ring), 1.60 (t, 2H, NHCH2CH), 3.05 (hump, 1H, CH2NH exchangeable with D2O). IR (cm-1, KBr): 1668 (C=N),3188 (NH of indole), 1740, 1720, 1710 (C=O), 717 (C-Cl), 1616 (C=C), 3045 (aromatic CH), 1585 (C-C of aromatic ring), 1485 (C-N), 1228 (OCH3), 690 (C-S-C).
General procedure to synthesize 4-(2’-oxo/thiobarbiturinyl acid) – 2 – (2”-halo-1”H-indolyl) – 3 – (substitutedphenyl aminomethylene) – 2,3 – dihydro – 1,5-benzoxazepines (23-30)
4-(2’-Oxo / thiobarbiturinyl acid) – 2 – (2”-halo-1”H-indolyl) – 2,3 – dihydro – -1,5-benzoxazepines (11-14) (0.001 mole), formaldehyde (0.01 mole) and anilines having different substitutions (0.001 mole) were dissolved in methanol (50 ml) to obtain clear solutions. The solution thus obtained were refluxed for about 5-6 hours. The reaction mixtures formed after refluxing were first evaporated, then chilled and finally put onto ice. After pouring onto ice semisolid products separated out. These semisolids were kept in petroleum ether (40-600 C) and were left overnight. The solid products were obtained the next day which were recrystallized using suitable solvents. The analytical as well as physical details of the compounds (23-30) are mentioned in Table-1. Compound 23: 1H-NMR CDCl3 δ: 5.66 (m, 1H, CH of barbituric acid), 9.16 (ss, 2H, 2NHCO), 9.85 (brs, 1H, N-H of indole), 7.62-6.82 (m, 12H, Ar-H), 3.48 (s, 3H, OCH3), 6.62 (s, 1H, C2-Hof oxazepine ring), 7.48 (d, 2H, C3-H of oxazepine ring), 4.25 (t, 1H, C4-H of oxazepine ring), 1.62 (t, 2H, NHCH2CH), 3.10 (hump, 1H, CH2NH exchangeable with D2O). IR (cm-1, KBr): 1730, 1725, 1720 (C=O), 720 (C-Cl), 1618 (C=C), 1582 (C-C of aromatic ring), 3184 (N-H of indole), 3048 (aromatic C-H), 1666 (C=N), 1482 (C-N), 1232 (OCH3), 1035 (C-O-C).
Pharmacology
Anticonvulsant activity
Supra maximal electroshock seizure pattern test (SMES) model was used for screening antiepileptic activity. Method of Tomen et al 27 was utilized for this model. Albino rats having weight ranging between 90-120 g of either sex were put to use for test. The rats were splited into groups. Groups were made in such a way that each group of rats contains ten animals. Test drugs as well as the standard drug – phenytoin sodium were injected intraperitoneally (i.p.) in rats. After 1 hour of drug administration, the rats were put through a shock of 150 milliampere via ear electrodes for 0.2 seconds. The absence or presence of extensor response in the rats was duly observed. Rats (animals) in which extensor reciprocation was put to end were taken as secured rats.
Acute Toxicity (ALD50)
The acute toxicity studies were carried out in mice. All the compounds which were prepared were scrutinized for acute toxicity (ALD50) in mice. This test was done by using the plan opf action given by Smith 28.
Results
Anticonvulsant activity in rats
All the compounds (1-30) synthesized were studied for their antiepileptic activity. The novel compounds were synthesized according the routes depicted in Figure-1. The compounds were tested for their biological activity i.e. antiepileptic test at a dose of 30 mg/kg i.p. against SMES experimental model (supra maximal electroshock induced seizures). The compounds 1-30 showed substantive activity varying between 40% to 90%. Out of thirty compounds evaluated, compounds 9, 20 and 28 exhibit excellent protection against convulsions thereby providing 80%, 90% and 80% protection against convulsions, correspondingly. The outcome of antiepileptic test are stated in Table-2.
The peculiarity of the compounds synthesized in this series is the presence of benzothiazepione/benzoxazepine nuclei on 2-thio/oxobarbituric acids (at the fifth position )containing indolyl moiety into a single molecular skeleton. The first step compounds i.e. 5-methoxy-2-oxo / thiobarbituric acids (1-2) showed mild (40%) antiepileptic effect. Going to the second step compounds i.e. 1-(2’-oxo / thiobarbiturinyl acid)-3-(2”-halo-1”H-indolyl)-prop-2-en-1-ones (3-6) a small increase in percentage protection was observed which was found ranging between 40% to 60%.
The next step of the series i.e. compounds 4-(2’-oxo / thiobarbiturinyl acid) – 2 – (2”-halo-1”H-indolyl) – 2,3 – dihydro – -1,5-benzothiazepines (7-10) and 4-(2’-oxo / thiobarbiturinyl acid) – 2 – (2”-halo-1”H-indolyl) – 2,3 – dihydro – -1,5-benzoxazepines (11-14) were prepared following path-1 and path-2 depicted in Figure-1. These compounds i.e. compounds (7-10) and (11-14) were found to possess good protection varying from 50% to 80% and 50% to 90% respectively in SMES model. The most potent compound of (7-14) is compound 9, which showed activity as good as reported by phenytoin sodium ( which was used as standard drug ) i.e. possessing similar protection of 80% as. Due to its potential character, it was further considered thoroughly at three graded and different doses of 7.5, 15 and 30 mg/kg i.p. for its antiepileptic profile and was found to have 30%, 60% and 80% protection. The compounds 4-(2’-oxo/thiobarbiturinyl acid) – 2 – (2”-halo-1”H-indolyl) – 2,3 – dihydro – -1,5-benzothiazepines (7-10) and 4-(2’-oxo / thiobarbiturinyl acid) – 2 – (2”-halo-1”H-indolyl) – 2,3 – di-hydro – -1,5-benzo-thiazepines (7-10) were further incorporated with various substituted anilines via Mannich reaction to yield compounds 4-(2’-oxo / thiobarbiturinyl acid) – 2 – (2”-halo-1”H-indolyl) – 3 – (substitutedphenyl aminomethylene) – 2,3 – dihydro – 1,5-benzothiazepines (15-22) and 4-(2’-oxo/thiobarbiturinyl acid) – 2 – (2”-halo-1”H-indolyl) – 3 – (substitutedphenyl aminomethylene) – 2,3 – dihydro – 1,5-benzoxazepines (23-30) respectively, which elicited good percentage protection against convulsion. Compounds (15-22),which were final compounds prepared via route-1 showed remarkable anticonvulsant activity differing from 60% to 90% whereas compounds (23-30) which which were final compounds synthesized via route-2 also showed unusual activity varying between 60% to 80%. Compound 20 was found to be the most potent compound among all the final stage compounds (15-30). This compound 20, as well as other potent compound 28 were also considered in details at three different and graded doses of 7.5 mg/kg i.p., 15 mg/kg i.p. and 30 mg/kg i.p. The results elicited that both of these compounds (20 and 28) showed same percentage protection of 40% and 60% at lesser doses of 7.5 and 15 mg/kg i.p. respectively. But a different percentage of protection against seizures was observed at a higher dose of 30 mg/kg i.p. (90% and 80% by compounds 20 and 28 respectively).
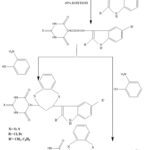 |
Figure 1 Click here to View Figure |
Conclusion
If we consider the anticonvulsant activity observed in all the newly synthesized compounds, it may concluded that-
Compounds containing thiobarbituric acid were proved to be better antiepileptic agents than the compounds having barbituric acid.
Benzothiazepine containing compounds were found to be more potent anticonvulsant agents than the benzoxazepine containing compounds.
Existence of electronegative atoms (chlorine and bromine) were found to increase the anticonvulsant effect in the compounds.
Presence of 2-chloro phenyl moiety at second position of benzothiazepine / benzoxazepine moiety elicited more promising effect than the 2-bromophenyl containing benzothiazepines/benzoxazepines.
Acute toxicity (Studied in mice)
All the compounds (1-30) synthesized were also evaluated for ALD50 (approximate lethal dose). They all were found to possesss excessive and good value of ALD50 greater than 1000 mg/kg i.p. Compound 20 showed exceptionally higher ALD50 greater than 2000 mg/kg i.p. Such higher ALD50 suggests the safer nature of these compounds. Table-2, contains the data of acute toxicity studies of these compounds.
Acknowledgement
Dr Archana, one of the author is grateful to U.P. govt., who has provided the financial aid by sanctioning the research project. This research project was sanctioned under the “Research and Development (R and D) Scheme of Uttar Pradesh for the teachers of state university and affiliated colleges.
Conflict of interest
The co-authors of this work declare and agree with the contents and there is no financial or ant other conflict of interest among them to report.
References
- Loscher, W. New versions in the pharmacology of anticonvulsants. Eur. J. Med. Pharm. 1998, 342, 1-3.
CrossRef - Leppik I.E. Treatment of epilepsy in the elderly. Current Treatment Options in Neurology. 2008, 10, 239-245.
CrossRef - Chen, L.; Sun, X.Y.; Chai, K.Y. Synthesis and aqnticonvulsant evaluation of 4-(4-alkoxyphenyl)-3-ethyl-4H-1,2,4-triazoles as potent open chain analogues of 7-alkoxy-4,5-dihydroxy[1,2,4]triazolo [4,3-a] quinolones. Bioorg. Med. Chem. 2007, 15, 6775-6781.
CrossRef - Drugs effective in the therapy of epilepsies. Goodman & Gilmans “ The pharmacological basis of therapeutics”, Mc Graw-Hill Co., New York 1996, 9th edition, 472.
- Drugs effective in the therapy of epilepsies. Goodman & Gilmans “ The pharmacological basis of therapeutics”, Mc Graw-Hill Co., New York 1996, 9th edition, 471.
- Shiradkar, M.R.; Ghodake, M.; Bothara, K.G.; Bhandari, S.V.; Nikhalje, A.; Akula, K.C.; Desai, N.C.; Burange, P.J. Synthesis and anticonvulsant activity of clubbed thiazolidinone- barbituric acid and thiazolidinone- triazole derivatives. ARKIVOC, 2007, 14, 58-74.
CrossRef - Kesharwani, S.; Sahu, N.K.; Kohli, D.V.; Synthesis and biological evaluation of some new spiro derivatives of barbituric acid. Pharm. Chem. J. 2009, 43, 315.
CrossRef - Goel, B.; Sharma, S.; Bajaj, K.; Bansal, D. Synthesis and CNS depressant activity of newer spirobarbiturates. Indian J. Pharm. Sci. 2005, 67, 194-199.
- Osman, A.N.; Kandeel, M.M.; Ahmed, M. Synthesis and anticonvulsant activity of some spiro compounds derived from barbituric and thiobarbituric acids. Indian J. Chem. 1996, 35B, 1073-1078.
CrossRef - Agarwal, A.; Lata, S.; Saxena, K.K.; Srivastava, V.K.; Kumar, A. Synthesis and anticonvulsant activity of some potential thiazolidininyl-2-oxo/thiobarbituric acids. Eur. J. Med Chem. 2006, 41, 1223-1229.
CrossRef - Archana; Srivastava, V.K.; Kumar, A.; Synthesis of some newer derivatives of substituted quinazolinyl-2-oxo/thiobarbituric acid as potent anticonvulsant agents. Bioorg. & Med. Chem. 2004, 12, 1257-1264.
CrossRef - Tyagi, M.; Archana. Synthesis and pharmacological evaluation of newer substituted 2-oxo/thiobarbiturinylbenzoxa/thiazepine derivatives as potent anticonvulsant agents. Oriental J. Chem. 2015, 31, 121-132.
CrossRef - Kerzare, D.R.; Menghani, S.S.; Rarokar, N.R.; Khedekar, P.B. Development of novel indole-linked pyrazoles as anticonvulsant agents: A molecular hybridization approach. Arch. Pharm. (Weinheim). 2021, 354, 1-21.
CrossRef - Swathi, K.; Sarangapani, M. Evaluation of anti-epileptic effect of new indole derivatives by estimation of biogenic amines concentrations in rat brain. Adv. Exp. Med. Biol. 2017, 988, 39-48.
CrossRef - Archana; Saini, S. Synthesis and anticonvulsant studies of thiazolidinine and azetidinone derivatives from indole moiety. Drug Res. 2019, 69, 445-450.
CrossRef - Hashemi, S.M.; Emami, S.; Masihi, P.H.; Shakiba, A.; Dehestani, L.; Ahangar, N. Synthesis of 2-aryl-3-triazolyl-indoles from phenacyltriazole-derived hydrazones : Exploring new scaffolds for anticonvulsant activity. J. Mol. St. 2023, 1276, 134704-134710.
CrossRef - Meng, Q.; Ren, X.; Wang R.; Han, Y.; Li, X.; Zhang, Q.; Li. Z.; Wang, Y.; Huang, L.; Yu, H. Design, synthesis, anticonvulsant activity and structure-activity relationships of novel 7-azaindole derivatives. Bioorg. Chem. 2023, 133, 106430.
CrossRef - Kendre, B.V.; Landge, M.G.; Bhusare, S.R. Synthesis and biological evaluation of some novel pyrazole, isoxazole, benzoxazepine benzothiazepine and benzodiazepine derivatives bearing an aryl sulfonate moiety as antimicribiual and anti-inflammatory agents. Arabian J. Chem. 2019, 12, 2091-2097.
CrossRef - Ayyash, A.N.; Fadel, E.J. Design, synthesis and antimicrobial studies of novel derivatives : benzoxazepine-4,7-dione and benzodiazepine-4,7- dione. Indian J. Heterocyl. Chem. 2019, 29, 255-259.
- Neochoritis, C.G.; Tsoleridis, C.A.; Stephanidou-Stephanatou, J.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.J. 1,5-Benzoxazepines vs 1,5-Benzodiazepines. One-pot microwave-assisted synthesis and evaluation for antioxidant activity and lipid peroxidation inhibition. J. Med. Chem. 2010, 53, 8409-8420.
CrossRef - Fiore, D.; Proto, M.C.; Pistani, S.; Picardi, P.; Zottola, C.P.; Butini, S.; Gemma, S.; Casagni, A.; Laezza, C.; Vitale, M.; Ligresti, A.; Marzo, V.D.; Zistere, D.M.; Nathwani, S.; Williams, D.C.; Campiani, G.; Gazzerro, P. Antitumor effect of pyrrolo-1,5-benzoxazepine-15 and its synergistic effect with oxaliplation and 5-FU in colorectal cells. Cancer Biology & Therapy 2016, 7, 849-858.
CrossRef - Campiani, G.; Butini, S.; Gemma, S.; Nacci, V.; Fattorusso, C.; Catalanotti, B.; Giorgi, G.; Cagnotto, A.; Goegan, M.; Minnini, T.; Minetti, P.; Cesare, M.A.D.; Mastroianni, D.; Scafetta, N.; Galletti, B.; Stasi, M.A.; Castorina, M.; Pacifici, L.; Ghirardi, O.; Tinti. O.; Carminati, P. Pyrrolo[1,3]benzothiazepine-based atypical antipsychotic agents. Synthesis, structure-activity relationship, molecular modeling and biological studies. J. Med Chem. 2002, 45, 344-359.
CrossRef - Kaur, H.; Kumar, S.; Chaudhary, A.; Kumar, A. Synthesis and biological evaluation of some new substituted benzoxazepine and benzothiazepine as antipsychotic as well as anticonvulsant agents. Arabian J. Chem. 2012, 5, 271-283.
CrossRef - Tyagi, M.; Archana. Synthesis and pharmacological evaluation of newer substituted 2-oxo/thiobarbiturinylbenzoxa/thiazepine derivatives as potent anticonvulsant agents. Oriental J. Chem. 2015, 31, 121-132.
CrossRef - Garg, N.; Chandra, T.; Archana; Jain, A.B.; Kumar, A. Synthesis and evaluation of some new substituted benzothiazepine and benzoxazepine derivatives as anticonvulsant agents. Eur. J. Med. Chem. 2010, 45, 1529-1535.
CrossRef - Bajaj, K.; Archana; Kumar, A. Synthesis and pharmacological evaluation of newer substituted benzoxazepine derivatives as potent anticonvulsant agents. Eur. J. Med. Chem. 2004, 39, 369-376.
CrossRef - Tomen, J.E.P.; Swinyard, E.A.; Goodman, L.S. Properties of maximal seizures and their alteration by anticonvulsant drugs and other agents. J. Neurophysiol. 1946, 9, 231-239.
CrossRef - Smith, Q.E. Pharmacological screening tests. Progress in medicinal chemistry 1. Butterworth; London, 1961, 1, 1-33.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










