Crystal Growth and Characterization of Urea Nitrate
S. Balamugundan1 , C. Vijayaraj1
, C. Vijayaraj1 , B.Ravindran2
, B.Ravindran2 and M. Mariappan1*
and M. Mariappan1*
1Department of Chemistry, Thiru.Vi.Ka. Govt. Arts College (Affiliated to Bharathidasan University), Thiruvarur, 610003, Tamil Nadu, India.
2Department of Physics, Thiru.Vi.Ka. Govt. Arts College (Affiliated to Bharathidasan University), Thiruvarur610003, Tamil Nadu, India.
Corresponding Author E-mail: mmari101@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400111
Article Received on : 04 Dec 2023
Article Accepted on : 15 Jan 2024
Article Published : 19 Jan 2024
Reviewed by: Dr. Aminullah A
Second Review by: Dr. Ang- Yang
Final Approval by: Dr. Ioana Stanciu
Single crystals of urea nitrate have been grown from aqueous solution by slow evaporation technique. Powder diffraction analysis confirmed that the grown crystals belong to orthorhombic system. The presence of functional groups was confirmed by using (FTIR) spectroscopy. Optical absorption studies show very low absorption in entire visible region and the UV cut-off is found to be around 300 nm. Thermal analysis studies were carried out using TG/DSC analysis and the grown crystal is stable up to 156.4◦C. SEM and EDAX analysis was observed and the elementary composition and morphological changes were studied.
KEYWORDS:FT-IR; EDAX; SEM; TGA/DSC; UV-Vis-NIR; Urea nitrate
Download this article as:| Copy the following to cite this article: Balamugundan S, Vijayaraj C, Ravindran B, Mariappan M. Crystal Growth and Characterization of Urea Nitrate. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Balamugundan S, Vijayaraj C, Ravindran B, Mariappan M. Crystal Growth and Characterization of Urea Nitrate. Orient J Chem 2024;40(1). Available from: https://bit.ly/3SpWVLh |
Introduction
Expectation of the technological world is to fabricate bulk sized high quality single crystals to satisfy the optical applications in various fields 1. Necessity of NLO crystal arises to satisfy the device fabrications to fulfill the following applications for the improvement of technical side such as color display, frequency conversion, optical signal processing, inertial confinement fusion research in high energy lasers, electro optic switches, fiber optic data communication storage etc., Large number of research and investigations have been made for the past few decades due to the optical potential applications of the NLO crystals. On the invention of new type of NLO crystals several parameters were taken into considerations which results in the modification of optical, physical and electrical properties of the available NLO materials by the inclusion of dopants or by the incorporation of functional groups 2,3 for the purpose of fabrication made applications. Enhancement of some physical properties such as SHG efficiency, growth rate, laser damage threshold, optical transparency etc., Perfection of crystals were observed and recorded previously 4-7. Investigation of Urea confirms the very interesting NLO behavior towards the optical behavior nonlinear applications 8. Even the crystallization is very complicated for Urea but fruitful outcome have been achieved by taming some efforts on solution and vapor growth. The physical and optical properties of Urea are very much comparable with KDP and ADP crystals which were proven earlier 9. Derivatives of Urea are widely used in supramolecular chemistry and crystal engineering due to its versatility in synthesis and design of solid state structures and functional materials 10,11. Urea creates strong and stable hydrogen bond with organic acids other than mineral acids 12-15. Urea crystals attract the attention of both theoreticians and experimentalists due to the NLO piezoelectric properties. Urea is representative of one class of materials, which applicable to photonics and reference material in the DMOS (diffusive mixing of organic solution) experiment in microgravity carried out by NASA 16. New NLO frequency conversion materials have a significant impact on laser technology 17. The new search and for new and efficient NLO materials has resulted in the development of new class of materials called organic single crystals. In this paper, urea nitrate single crystal is synthesized and grown by slow evaporation technique at room temperature.
Synthesis
Distilled water of 36 ml quantity is taken in a 400ml beaker to dissolve 14.4g of Urea. The dissolved solution is made to stir using magnetic stirrer and 8.1 ml of Nitric acid was added by drop by drop while stirring. Immediate crashing of a white precipitate is formed from the solution. The obtained mixture was allowed to stir after the addition of nitric acid completely at 25°C for about 20 min. Finally the precipitate formed was washed with cold water (20 ml). A wet material of 4.8g weight was harvested from the above said treatment. Additionally a voluminous white precipitate was appeared when the mother liquid was then cooled to 0°C using an ice bath with an addition of 8.6ml nitric acid was added by drop by drop. The obtained solution mixture was filtered with a filter paper (Whatman) and the harvested cake was washed with 10 ml of cold ice water and then the harvested filtrates (both) were combined. The white powder was dried for about 4 hours using a vacuum over at 45°C which results in the formation of 18.2g of white powder. 10g of Urea nitrate was taken in a beaker to remove insoluble impurities and dissolved with double distilled water and stirred well with a magnetic stirrer. Then the solution was filtered using 10 micro meter paper sized Whatman filter paper. The filtered solution was kept isolated with a perforated lid for the controlled evaporation rate to get perfect crystallization at room temperature. After 8 days a well defined crystal was harvested by slow evaporation solution growth method. The grown crystal Physical morphology is shown in Fig.1.
Experiment
A single crystal of urea nitrate was prepared at room temperature by slow evaporation of aqueous solution containing equimolar proportion of urea and nitric acid. Care was taken to minimize mechanical and thermal variations. Colorless, bright and transparent crystal with average size of 2 X 1 X 0.8 cm was obtained. The crystal characterized as follows; TG-DSC of urea nitrate was carried out using a NETZSCH STA 409C thermal analyzer in the nitrogen atmosphere. SHIMAZU 6000 X-ray diffractometer is used to record the powder XRD pattern for urea nitrate. A Bruker IFS 66V spectrometer was used to record FTIR spectra of the compound, employing KBr pellet technique in the frequency range 400-4000 cm-1. The spectrophotometer Perkin Elmer Lambda 35 was used to record the UV-Vis-NIR spectra between the range 200-1100 nm. Scanning Electron Microscope study was taken for the sample using JSM6390. Finally INCA PENTA FET X3 was used to carry out the EDAX analysis.
 |
Figure 1: Morphology of Urea nitrate crystals. |
Fig.2 shows the UV-Vis-NIR spectrum of urea nitrate which was a highly transparent single crystal. From the absorption spectrum it was confirmed that the grown crystals are highly transparent in the entire 300-1100nm and shows no absorption peak which will be a needful parameter for a potential NLO material. The absorption percentage in the entire visible region confirms that it is the desirous property for NLO applications. Further observations in the UV-Vis-NIR spectra shows that the grown crystal may be used to utilize in the blue region frequently with laser.
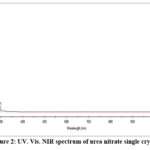 |
Figure 2: UV. Vis. NIR spectrum of urea nitrate single crystal. |
FT-IR spectrum
The urea nitrate functional groups were identified using FTIR spectrum analysis. The presence of -CO is confirmed from the aroused higher band 1707cm-1 and -NO3 was confirmed from the lower cutoff band at 1319cm-1. The symmetric and asymmetric stretching vibrations of Urea were found at 3414cm-1 and 3368cm-1 respectively. The band found at 2428cm-1 was due to -OH symmetric stretching of urea nitrate from hydrogen bonding of adjacent C=O—–HNO2 urea nitrate molecules which will expose the confirmation about the presence of nitrate in urea nitrate crystals.
 |
Figure 3: FTIR spectrum of urea nitrate. |
Thermal Studies
A variety of analytical methods were used to grow 21g of urea nitrate with respect to literature methods of high purity [13-15]. The decomposition and melting point of employed TG/DSC results are used to verify the purity of the sample. The obtained melting point of the grown sample was found to be noted 156.4°C along a decomposition of 162°C which has a very good agreement with the reported values between 152°C as melting point and the decomposition as 162°C from literature.
To evaluate vacuum stability as well as the magazine potential storage conditions the optimum temperature was determined by thermogravimetry analysis with various temperature range. The samples were run between 30°C to 800°C first by ramping at 10°C/min temperature. The weight loss took place at 140°C and ends at 254.72°C for urea nitrate approximately. A 90% weight loss was happened for the above range of temperature and this is due to the liberation of NH3, CO2 and HCNO.
 |
Figure 4: TG-DSC of urea nitrate. |
X-ray diffraction analysis
The X-ray diffraction of urea nitrate is shown in Fig. 5. It is shown that the number of planes absorbed decreased and d values is also decreased. This may be due to the overlapping of planes of urea crystals and nitrate there by there in reorientation in the structure and this brings at different morphology of the crystals itself. This is very much striking phenomena observed and this mixed combination of urea and nitrate will produce a wide range of laser active crystals. The powder XRD reveals the orthorhombic system with a space group P212121.
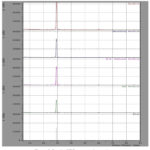 |
Figure 5: Powder XRD pattern of urea nitrate. |
SEM analysis
The resolution SEM can be much higher than that of an optical microscope. Surface analysis of the grown urea nitrate on a JSM6390 showed a step like structure on the surface of urea nitrate which confirm the layer growth of urea nitrate. It is seen that the crystallite size increases due to compound formation and the samples crack free with well defined grain boundaries.
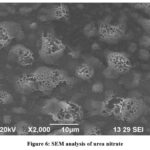 |
Figure 6: SEM analysis of urea nitrate. |
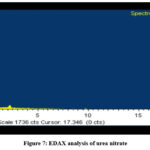 |
Figure 7: EDAX analysis of urea nitrate. |
EDAX Analysis
The EDAX analysis of urea nitrate is shown in Fig.7. The higher percentage of nitrogen and oxygen in urea when compared to urea indicates the inclusion of nitrate in urea.
Conclusions
Transparent single crystals of urea nitrate were successfully grown by slow evaporation technique at room temperature. The powder XRD reveals the orthorhombic system with a space group P212121. Optical behaviors of the present crystal were studied by using UV-Vis-NIR spectrometer and found that the lower cut-off wavelength is 300 nm which is suitable for optical applications. Functional groups and the presence of all elements of the urea nitrate crystal were confirmed by FT-IR and EDAX test respectively. Thermal stability and melting point of the title compound were accessed by TG-DSC analysis.
Acknowledgement
We thankful for St. Joseph’s College in Tiruchirappalli for providing the FTIR and UV visible, research for the centralized instrumentation facilities laboratory. Karunya University in Coimbatore has a lab with equipment for XRD and SEM study results with EDAX. National Institute of Technology, Tiruchirappalli for proving TG-DSC.
Conflict of Interest
The authors declare no competing interests.
Funding Sources
There is no funding sources
References
- Riscob, B.; Shakir, M,; Vijayan,N,; Maurya,K.K,; Wahab,M.A,; Bhavannarayana,G,; J. Appl. Cryst.,2012, 45, 679. https://doi.org/10.1107/S0021889812016822
CrossRef - Madhurambal,G,; Ravindran,B,; Mariappan,M,; Ramamurthi,K,; Mojumdar,S.C,; J Therm Anal Calorim., 2011, 104 (3) 875. https://doi.org/ 10.1007/s10973-011-1300-8
CrossRef - Mojumdar,S.C,; Madhurambal,G,; Mariappan,M,; Ravindran,B,; J. Therm.Anal. Calorim., 2011,104 (3): 901. https://doi.org/10.1007/s10973-011-1490-0
CrossRef - De Vries, S.A,; P., Goedtiindt, S.L,; Bennett, W.J,; Huisman,M.J,; Zwanenburg, M.J,; Smilgies,D.M,; De Yoreo,J.J,; Van Enckevort, W.J.P,; Bennema, P,; Vlieg, E,; Phys Rev Lett., 1998,80:2229. https://doi.org/10.1103/PhysRevLett.80.2229
CrossRef - Kar, S,; Bhatt,R,; Bartwal,K.S,; Wadhawan, V.K,; Cryst Res Technol., 2004,39:230. https://doi.org/10.1002/crat.200310175.
CrossRef - Winkler,E,; Etchegoin,P,; Fainstein, A,; Fainstein, C,; Phys. Rev B., 2000, 61:15756. https://doi.org/10.1103/PhysRevB.61.15756
CrossRef - Bhagavannarayana,G,; Parthiban, S,; Meenakshisundaram,S,; Cryst. Growth. Des., 2008, 8:4462008. https://doi.org/10.1021/CG0702129
CrossRef - Madhurambal,G,; Mariappan, M,; Ravindran,B,; Mojumdar,S.C,; J.Therm. Anal. Calorim., 2011, 104(3),885. http://doi.org/10.1007/s10973-011-1290-6
CrossRef - Ardoino,M,; Zeng,L,; Razzetti Zha,C,; Zanotti,L,; Curti,M,; Mater Chem Phys., 2000, 66:299. http://dx.doi.org/10.1016/S0254-0584(00)00306-0
CrossRef - Zhao, X,; Chang,Y,; Fowler,F,; Lauher,J,; J. Am. Chem. Soc., 1990, 112;6627. https://doi.org/10.1021/ja00174a026
CrossRef - Madhurambal,G,; Mariappan, M,; Selvarajan,G,; Mojumdar,S.C,; J.Therm. Anal. Calorim., 2015,119 (2), 931. http://doi.org/10.1007/s10973-014-4249-6
CrossRef - Mariappan,M,; Madhurambal,G,; Ravindran, B,; Mojumdar,S.C,; J. Therm. Anal. Calorim., 2011,104 (3), 915. http://dx.doi.org/10.1007/s10973-011-1293-3
CrossRef - Ravindran,B,; Madhurambal,G,; Mariappan,M,; Mojumdar, S.C,; J Therm. Anal Calorim.2011,104(3): 893. https://doi.org/10.1007/s10973-011-1291-5
CrossRef - Oxley,J.C,; Smith,J.L,; Naik.S,; Moran.J,; J. Energ. Mater., 2009, 27:17. https://doi.org/10.1080/07370650802328814
CrossRef - Dayana Jeyaleela, G,; Rosaline Vimala, J,; Senthil, R,; Anandagopu, P,; Manjula, K,; Asian J. Chem., 2019, 31(11), 2628-2634. https://doi.org/10.14233/ajchem.2019.22217
CrossRef - Boomadevi, S,; Dhanasekaran, R,; Ramasamy, P,; Cryst. Res. Technol. 2002, 37, 159. https://doi.org/10.1002/1521-4079. (200202)37:2/3%3C159::AID-CRAT159%3E3.0.CO;2-Y
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









