Efficient Adsorption of Trivalent Rare Earth Elements Using Nano Copper Ferrite : Kinetics, Isotherms and Thermodynamics
Sudha Thotakura1, Anantha Lakshmi Vadivelu1, Sivaji Maganti1 , Rama Murthy Duvvuri2 and Suryakala Duvvuri1*
, Rama Murthy Duvvuri2 and Suryakala Duvvuri1*
1Department of Chemistry, GITAM Institute of Science, GITAM University (Deemed to be), Visakhapatnam-530 045, Andhra Pradesh, India.
2Department of Chemistry, Government Degree College (A),Tuni, Andhra Pradesh, India.
Corresponding Author E-mail: duvvurisuryakala@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390628
Article Received on : 28 Oct 2023
Article Accepted on :
Article Published : 19 Dec 2023
Reviewed by: Dr. M K Raman
Second Review by: Dr. Munther Al-Amery
Final Approval by: Dr. Balkrishan
Rare earth elements (REEs) have versatile applications in various industries and are thrown as industrial waste waters, which leads to serious environmental issues. This paper describes the adsorption efficiency of nano copper ferrite on REEs namely lanthanum and cerium in their trivalent states at room temperature and at the optimum conditions of pH, contact time, adsorbent, adsorbate dosage, temperature etc. The batch adsorption studies were conducted for the adsorption studies using ICPAES (Inductive coupled plasma atomic emission spectro photometer). In addition the data is studied for Langmuir and Freundlich models and observed that data well fitted to Langmuir monolayer adsorption. From the graph and intercept values of theoretical and experimental values, the pseudo second order kinetics are followed. Thermodynamic parameters reveal that reaction is spontaneous and endothermic . From the over all observations like fast kinetics, chemical stability, high adsorption capacity it is concluded that nano CuFe2O4 has maximum efficiency for the adsorption of REEs.
KEYWORDS:Adsorption; Langmuir Adsorption Isotherms; Nano Spinel Copper Ferrite; Trivalent Rare Earth Elements; Thermodynamic Parameters
Download this article as:| Copy the following to cite this article: Thotakura S, Vadivelu A. L, Maganti S, Duvvuri R. M, Duvvuri S. Efficient Adsorption of Trivalent Rare Earth Elements Using Nano Copper Ferrite : Kinetics, Isotherms and Thermodynamics. Orient J Chem 2023;39(6). |
| Copy the following to cite this URL: Thotakura S, Vadivelu A. L, Maganti S, Duvvuri R. M, Duvvuri S. Efficient Adsorption of Trivalent Rare Earth Elements Using Nano Copper Ferrite : Kinetics, Isotherms and Thermodynamics. Orient J Chem 2023;39(6). Available from: https://bit.ly/3ROnh9i |
Introduction
REEs consists of lanthanide series and scandium plus yttrium.They have 4f orbital as inner electronic configuration and outer shell has 5d1-10 and 6s2 electrons. This electronic configuration makes lanthanides more special for fast reactions. Their physical characteristic properties are almost close due to similar electronic configuration1-3.They have difference in chemical properties because of lanthanide contraction of ionic radius and electrostatic effect associated with increase of shielded nuclear charge through electrons. The trivalent form {M(III)}is the most thermodynamic stable form4-6. Lanthanides have many applications in various fields such as super conductors, fertilizers, Lasers, catalysts and optical fibers. As they are unable to pass through cell membrane, they are non toxic.In the present work La(III) and Ce(III) are selected which are from light REEs family7.
Lanthanum is the 57th element in the periodic table,it causes nervous system disorder in humans. Also the petroleum industry waste, which has lanthanum, dumped into waste waters.This causes severe damage to plants and animals8. The atomic number of cerium is 58 and the pure metal itself can ignite if scratched with knife. It is strong reducing agent and causes itching, sensitivity to heat and skin lesions are experienced9.The main interest in selecting La(III)and Ce(III)is that they are 80% global consumption and also present in industrial waste water.The main aim is to obtain a potential adsorbent for REE extraction from aqueous media.
Till date several methods are applied for the extraction of REE mainly using solvent extraction10, electro chemical precipitation11, Ion exchange12 and adsorption13. Among them adsorption is preferred due to its reversibility, eco friendly nature, cheap, simplicity and hence applicable for the REE ions in aqueous samples14.However the investigation of REE from aqueous solution is limited. Adsorption is treated as efficient, quick and low cost procedure in the recovery of metals 15.
Nano spinel copper ferrites are used for the adsorption of REE from aqueous solution. As these spinel ferrites are famous for their thermal stability, chemical and magnetic properties.They are widely used as catalysts, magnetic fluids and microwave radiation isolators16. Nano spinel copper ferrite is prepared using co-precipitation method which is most widely used in the synthesis of ferrite nano materials. The nano particles are prepared by using the procedure explained Suryakala et al., and characterised as per analytical techniques17. These copper ferrites have tetragonal structure and are more stable at room temperature. Due to their thermal, chemical stabili ty and low toxicity these materials are used as adsorbents in the adsorption of above said REEs18.
Experimental
All chemicals used are of analytical grade.The chemicals like Lanthanum nitrate, Cerium nitrate ,NaOH, HCl are purchased from Qualigen’s chemicals and glass ware. Solutions are prepared using deionised water (18.2M Ώ Cm). Solutions are prepared just before the usage. The nano spinel copper ferrite is synthesized using co precipitation method. In Coprecipitation the solutions are mixed directly without further time delay. It is an eco friendly procedure. Due to simplicity and high yield co-precipitation method is most widely used methods in the synthesis of magnetic nanoferrites. Co-precipitation is simple with high crystalline and textural properties.
Synthesis of CuFe2O4:
Aqueous solution of iron nitrate Fe(NO3)3.9H2O and copper nitrate Cu(NO3)3.6H2O with stoichiomeric ratio Cu: Fe = 1:2 were stirred for one hour (for each 1 gram of nitrate, 3.3 ml of milli Q water are used. 1molar NaOH solution is added, for each
1 gram of nitrate, followed by further stirring for 15 minutes. The material is annealed for 2 hours at 7000 C, at ordinary atmosphere in the muffle furnace. This is further characterized using analytical techniques. Concentration of REE metal ion in both initial and with drawn samples are calculated through ICP-AES technique.
Adsorption Experiments
The equilibrium adsorption experiments can be performed individually at room temperature. The batch experiments are carried out to evaluate the adsorption process and REE mobilization. Every time a particular parameter is varied by fixing all other parameters at their optimum values and the effect of temperature, kinetics,isotherms and thermodynamic parameters are calculated. Also the parameters like effect of pH, nano spinel ferrite (adsorbent) dose, REE’s dose, contact time are optimized. However % of REE adsorbed can be calculated using the formula given as
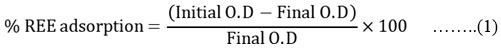
here O.D = optical density of REE .
Results and Discussion
Effect of pH
The pH is key factors during the adsorption process.The amount of REE’s adsorption onto nano copper ferrite was studied at pH=2 to 11. Results showed that when pH was higher than 7, the adsorption capacity was maximum. At lower pH, the adsorption capacity was not maximum, but the results are in agreement with the observations of other researcher’s group19. At lower pH values the protons are in competition with adsorbates (REEs) and nano copper ferrites retain very small amount of lanthanides, as a result the adsorption of La(III) and Ce(III) is very less. Also at when pH is increased adsorbate – adsorbent electrostatic repulsion decreases and adsorbed Lanthanides seems stable20. A pH value of 7.8 is fixed as optimum for the entire study.
 |
Figure 1: pH effect on adsorption of REE on nano spinel copper ferrite |
Effect of contact time
Adsorption capacity increases with time can be explained in 2 steps. That is in first 20 minutes a steep elevation in the sorption. Around 95% of the overall sorption takes place with in first 30 minutes of contact time. Proper reaction rate , diffusion mechanism are very significant in explaining the role of contact time21.
 |
Figure 2: Contact time effect on adsorption of REE onto nano spinel copper ferrite. |
When all the sites on porous material of the adsorbent reach saturation, the maximum adsorption is completed. Hence figure 2 explains the effect of contact time at optimum pH and at room temperature whsen the adsorbent dose is 0.5 g. More over in basic medium REE ions are automatically precipitated as oxides, hydroxides,hence adsorption is significantly decreased22.
Adsorbent dosage ( nano spinel copper ferrite):
The nano spinel copper ferrite dosage is varied from 0.1 to 1gm under the optimum conditions of other parameters (pH= 7.8, contact time =30 min for both REEs and at room temperature). Relationship between nano spinel copper ferrite dosage and removal efficiency of the REE from their aqueous solutions is presented in figure 3. Nano spinel copper ferrite shows maximum removal efficiency of more than 90% ions at 500 mg. The rate of adsorption increases with dose of adsorbent is due to availability of more adsorption sites with further growth of surface area. This reduced with increase in adsorbent dose. Overlapping of adsorption sites as a result of overcoming of copper ferrite particles23. By increasing the initial adsorbent concentration in solution, the quantity of adsorbed lanthanides gradually increases. But increase starts to ease after 0.2 gm/L. Trend is probably because of the adsorption sites saturation at higher concentrations.
 |
Figure 3: Effect of adsorbent dosage on adsorption of REE on nano spinel copper ferrite. |
Dose of REEs
Effect of REEs dosage on adsorption can be done by preparing aqueous solution of lanthanum nitrate and cerium nitrate respectively. The range is from 5ppm to 30ppm. The dose of nano copper ferrite is 0.5 g throughout. The total volume is 30.0 ml. The temperature of the system is 300K with a pH of 7.8. The solution was centrifuged and the supernatent liquid concentration is observed using ICPAES, with time until the system approaches equilibrium24.The same procedure is repeated until the error is minimized and attains reproducibility.The maximum adsorption was observed at 10 and 8 ppm respectively for the REE ions. This is because, establishment of saturated more ionic layer at lower concentration of metal ions, makes extra interaction near the adsorbent hindered in high concentration solution due to the interactions among REE cations existence on the copper ferrite medium. Also tendency for an ion to remain in Stern layer depends on the electro porosity of the metal ion25.
 |
Figure 4: Dosage of La(III) on adsorption by nano spinel copper ferrite |
 |
Figure 5: Dosage of Ce (III) on adsorption by nano spinel copper ferrite. |
Kinetic models
To compare the efficiency of nano spinel copper ferrite at various conditions and concentrations, to design and optimise an operating procedure , the analysis of equilibrium data is very important, because the adsorption is a mass transfer operation and is elaborated by a rate process. However to examine the characteristics of adsorption process, kinetic data, potential rate controlling data are analyzed using pseudo first order and pseudo second order.They can be represented as
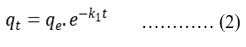
k1 is the rate constant for pseudo first order kinetics.
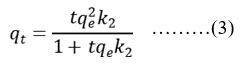
k2 is the rate constant for pseudo second order kinetics
From reports the kinetics of the elements do follow pseudo second order kinetics for both REEs. However R2 for pseudo second order at different concentrations are above 0.99. Adsorption is chemisorption through sharing of electrons among copper ferrite and adsorbate26. From this data it is suggested that adsorption rates are quick at lower concentrations for lanthanides. In other words time required for the equilibrium adsorption increases as RE ions concentration increases.
Table 1: parameters for PFO and PSO kinetics for the adsorption of REE on nano spinel copper ferrite.
|
PFO parameter |
La(III) |
Ce(III) |
PSO parameter |
La(III) |
Ce(III) |
|
Ci(mg/L) |
10 |
8 |
Ci(mg/L) |
10 |
8 |
|
K1 (min-1) |
0.026 |
0.024 |
K2(g mg-1min-1) |
2.45x 10-4 |
2.37x 10-4 |
|
qe (mg/g) |
119.6 |
110 |
qe (mg/g) |
116 |
112.4 |
|
R2 |
0.980 |
0.986 |
R2 |
0.996 |
0.996 |
 |
Figure 6: Pseudo first order kinetics for the adsorption of REE on nano spinel copper ferrite. |
 |
Figure 7: Pseudo second order kinetics for the adsorption of REE on nano spinel copper ferrite. |
Adsorption isotherm models
To establish equilibrium data of adsorption, the experimental data is studied with respect to Langmuir and Freundlich models. From the table 2, the experimental data is fitted for Langmuir model and hence adsorption is mono layer27. Due to the ionic radius closeness of La (III) = 1.051A0 and Ce (III) = 1.034A0. The qm values for REEs is almost equal. As the Langmuir model assumes mono layer adsorption the data fits more into this. The linearised form is
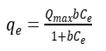
qmax = adsorption capacity (µg g-1) and b is energy of adsorption.
qe = kF Ce1/n
kF is adsorption capacity and “n” is adsorption intensity.
KL and qm are from linear fit of ce/qe vs ce. Langmuir model is the most widely used and covers monolayer adsoption. This model is appropriate with specific parameters for adsorption of REEs on copper ferrites28. Maximum mono layer adsorption capacities are found to be 333 and 345 mg/g for lanthanum and cerium respectively. However E values from 8 to 16 KJ/mol explain chemical adsorption.
 |
Figure 8: Langmuir adsorption isotherms for adsorption of REE by nano copper ferrite |
Table 2: Parameters for Langmuir, Freundlich models for the adsorption of REE on nano spinel copper ferrite
|
Isotherm model |
parameter |
La (III) |
Ce(III) |
|
Langmuir |
Q0 (mg/g) |
333 |
345 |
|
bL (L/mg) |
4.73 x10-2 |
2.09 x 10-2 |
|
|
R2 |
0.997 |
0.989 |
|
|
Freundlich |
KF (mg/g) |
20.6 |
9.82 |
|
nF |
1.78 |
1.40 |
|
|
R2 |
0.918 |
0.920 |
Effect of temperature and thermodynamic parameters:
The thermodynamic studies are more important to understand the mechanism of adsorption for the practical applications. To study these effects Gibb’s free energy and Van’t Hoff equation are followed.
ΔG0 = -RTlnKL
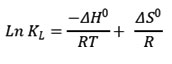
–ΔG0 is the change in Gibb’s free energy , R ideal gas constant (8.314J/mol/K), T absolute temperature (K), KL Langmuir constant. ΔH0,ΔS0 are changes in enthalpy and entropy respectively. Effect is examined on the adsorption of REE by nano copper ferrite at the range of 300K 310K and 320K at pH 7.8 and constant time of 60 minutes.The concentration of La (III) and Ce(III) are 10 and 8 ppm respectively at the concentration of adsorbent is at 0.5g.
Both the REE are temperature dependent and at higher temperature the increase in the dose of adsorbed lanthanides is because of the higher affinity of the available active sites. Positive values of enthalpy change (H0) decrease in the Gibb’s free energy change with rise of temperature encourages sorption mechanism is endothermic29.
More over positive values of entropy change (ΔS0) means disorder of the system increases after metal sorption. Increase of entropy is due to release of water during the metal sorption of RE ions. This fact that ΔH0 is less than TΔS0. This explains that sorption process is through entropy rather than enthalpic changes30.
 |
Figure 9: Temperature effect on adsorption of REE by nano copper ferrite. |
 |
Figure 10: Thermodynamic parameters for the adsorption of REE by nano spinel copper ferrite. |
Table 3: Thermodynamic parameters for La (III) and Ce(III) by adsorption of nano spinel copper ferrite
|
REE |
ΔH0 (KJ mol-1) |
ΔS0(J mol-1K) |
T(K) |
ΔG0 (KJ mol-1) |
TΔS0(KJ mol-1) |
|
La(III) |
16.0 |
138 |
300 |
-24.8 |
40.8 |
|
310 |
-25.6 |
41.2 |
|||
|
320 |
-27.1 |
42.8 |
|||
|
Ce(III |
11.0 |
126 |
300 |
-25.8 |
36.8 |
|
310 |
-27.0 |
37.6 |
|||
|
320 |
-28.3 |
38.0 |
Conclusions
Nano spinel copper ferrite is applied as capable adsorbent in the removal of REE from aqueous solutions. Present investigation helps to understand the greater efficiency of nano spinel copper ferrite in terms of maximum adsorption capacity of 983 and 973 mg/g for La(III) and Ce(III) respectively. The optimum conditions for the parameters are obtained. The pH of 7.8 is opted for both the elements to reach the equilibrium in 30 minutes. Langmuir isotherm model is fitted well followed by pseudo second order kinetics. The system is thermodynamically spontaneous, endothermic and randomness increases with temperature. Hence to conclude the adsorption is economical, environmental benign procedure.
Acknowledgement
Authors thank GITAM authorities for providing necessary conditions to conduct the work.
Conflict of Interest
Authors have no conflict of interest regarding work and publication.
References
- Wang,S.; Dong, Y.; He, M.; Chen,L.; Yu, X.Characterization of GMZ bentonite and its application in the adsorption of Pb(II) from aqueous solutions, Appl. Clay Sci. 2009,43, 164-171.
CrossRef - Sorace,L.; Benelli, C.; Gatteschi, D.Lanthanides in molecular magnetism: old tools in a new field, Chem. Soc. Rev. 2011,40, 3092-3104.
CrossRef - Wang,Q.; Song, J. Li.; Wang, X. Facile synthesis of high-quality plasma-reduced graphene oxide with ultrahigh 4,4′-Dichlorobiphenyl adsorption capacity,Chem. Asian J. 2013, 8, 225-231.
CrossRef - Eggert, R.;Wadia, C.; Anderson, C.; Bauer, D.; Fields, F.; Meinert, L.;Taylor,P.; Rare Earths: Market Disruption, Innovation, and Global Supply Chains, Annu. Rev. Environ. Resour, 2016, 41,199-212.
CrossRef - Zhao.Z.; X. Sun, Y. Dong, Synergistic Effect of Doped Functionalized Ionic Liquids in Silica Hybrid Material for Rare Earth Adsorption. Ind. Eng. Chem. Res, 2016,55,2221-2229.
CrossRef - Sun,X.; Waters, K.E.; Development of Industrial Extractants into Functional Ionic Liquids for Environmentally Friendly Rare Earth Separation, ACS Sustain. Chem. Eng. 2014, 2, 1910-1917.
CrossRef - Das, D.; Jaya Sre Varshini,C. ; Das, N. ; Removal and potential recovery of rare earth elements from mine water, Min. Eng. 2014,69,40-56.
CrossRef - Awwad,N.S.; Gad, H.M.H.;. Ahmad,M.I.; Aly, H.F. Sorption of lanthanum and erbium from aqueous solution by activated carbon prepared from rice husk, Colloids Surf. B Biointerfaces, 2010, 81, 593-599.
CrossRef - Yao, M. ;Joly, A.G. ;Chen, W. Formation and Luminescence Phenomena of LaF3:Ce3+ Nanoparticles and Lanthanide−Organic Compounds in Dimethyl Sulfoxide. J. Phys. Chem. C, 2010, 114 , 826-831.
CrossRef - Xie, F.; Zhang,T. A.;Dreisinger,D. ; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions, Miner. Eng, 2014,56,10-28.
CrossRef - Oshita,K.; Takayanagi,T. ; Oshima,M.; Motomizu,S. Adsorption behaviour of cationic and anionic species on chitosan resins possessing amino acids moieties, Anal Sci, 2007, 23, 1431-1434.
CrossRef - Zhang, G. ;Liu,H. ; Liu,; Qu, J.Adsorption behavior and mechanism of arsenate at Fe–Mn binary oxide/water interface, J. Hazard. Mater. 2009,168,820-825.
CrossRef - Madadrang,C.J. ; Kim, H.Y.;Gao, G.; Wang,N. ; Zhu,J.;Feng,H. ; Gorring, M. ; Kasner, M. L.;Hou, S. Adsorption behavior of EDTA-graphene oxide for Pb (II) removal, ACS Appl. Mater. Interfaces 2012, 4, 1186-1193.
CrossRef - Ali, I, New Generation Adsorbents for Water Treatment, Chem. Rev. 2012, 112 , 5073-5091.
CrossRef - Najafi, F.; Moradi, O.; Rajabi, M.; Asif, M.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Thermodynamics of the adsorption of nickel ions from aqueous phase using graphene oxide and glycine functionalized graphene oxide, J. Mol. Liq. 2015, 208, 106–113.
CrossRef - Upadhyay,R.V.; . Davies,K.J.; Wells, S.; Charles,S.W.; Preparation and characterization of ultra-fine MnFe2O4 and MnxFe1−xFe2O4 spinel systems: I. particles, J.Magn. Magn. Mater. 1994, 132 ,249-257.
CrossRef - Anantha Lakshmi ,V.; Sudha ,T.; Sivaji , M.;Vasundara ,D.; Suryakala , D.; synthesis and characterisation of MFe2O4 (M = Zn,Cu) spinel ferrite nano particles, Journal of Pharmaceutical Negative Results , 2022, 13(7),7650-7656.
- Reddy,L.H. Arias, J.L.Nicolas,J. Couvreur, P.; Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications, Chem. Rev. 2012, 112, 5818-5878.
CrossRef - Qiu ,H.;Lv , L.; Pan ,B.; Zhang ,Q. ; Zhang , ;Zhang,Q. ; Kinetic and Equilibrium Isotherms Studies of Adsorption of Pb(II) from Water onto Natural Adsorbent, J Zhejiang Univ Sci A 2009,10,716-724.
CrossRef - Qu ,Y.; Lian,B.; Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10, Bio resour Technol, 2013 ,136,16-23.
CrossRef - Roosen, J.;Binnemans, K.; Adsorption and chromatographic separation of rare earths with EDTA- and DTPA-functionalized chitosan biopolymers, J Mater Chem A ,2014, 2, 1530-1540.
CrossRef - Rahmati ,A. ;Ghaemi .A.; Samadfam M.; Kinetic and thermodynamic studies of uranium(VI) adsorption using Amberlite IRA-910 resin, Ann Nucl Energy, 2012, 39,42-48.
CrossRef - Pui,A.; Gherca,D. ; Cornei,N. ;Synthesis and characterization of MFe2O4 (M = Mg, Mn, Ni) nanoparticles, Mater. Res. Bull. 2013, 48,1357-1362.
CrossRef - Hosomomi ,Y. ; Baba, Y.;Kubota , F. ; Kamiya ,N.; Goto. M.,; Biosorption of Rare Earth Elements by Escherichia coli, J Chem Eng Jpn, 2013, 46, 450-454.
CrossRef - George Z. K.; Kostas A. M. ; Nanoadsorbents for pollutants removal: A review,J. Mol Liq,2015, 203,159-168
CrossRef - Han,R. ; Zhang, J.; Han, P.; Wang,Y.; Zhao,Z.; Tang,M. ; Kinetics of Adsorption of Lead onto Low Cost Adsorbents: Comparison of Linear and Non-Linear Regression Methods, Chem. Eng. J. 2009, 145, 496-504.
CrossRef - Gupta,A.K.; Gupta, M.;Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications, Biomaterials,2005, 26, 3995-4021.
CrossRef - Foo , K.Y.; Hameed, B.H.; Insights into the modeling of adsorption isotherm systems, Chem Eng J . 2010, 156,2-10.
CrossRef - Diakonov,I. ;Ragnarsdottir, K.V.; Tagirov, B.R.; Standard thermodynamic properties and heat capacity equations of rare earth hydroxides:: II. Ce(III)-, Pr-, Sm-, Eu(III)-, Gd-, Tb-, Dy-, Ho-, Er-, Tm-, Yb-, and Y-hydroxides. Comparison of thermochemical and solubility data, Chem. Geol. 1998,151, 327-347.
CrossRef - Anastopoulos,I.; Kyzas,G.Z.; Are the thermodynamic parameters correctly estimated in liquid-phase adsorption phenomena?, J. Mol. Liq. 2016, 218 ,174-185.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









