Design of Novel Thiohydantoin Derivatives and Exploration their Physico-Chemical Parameters
Prashant A. Gotmare* and Sanjay V. Kolhe1
and Sanjay V. Kolhe1
Department of Chemistry, Shri Shivaji Arts, Commerce and Science college, Akot, Dist.Akola-444101, (Maharashtra), India.
Corresponding Author E-mail: prashantpatilchem2763@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390520
Article Received on : 28 Aug 2023
Article Accepted on : 11 Oct 2023
Article Published : 18 Oct 2023
Reviewed by: Dr. Bull Okpara Sergeant
Second Review by: Dr. Muthulakshmi Andal
Final Approval by: Dr. Tawkir Sheikh
Thiohydantoin analogues was heterocyclic non-aromatic five membered cyclic compounds obtained from aurones derivatives. In this article, we synthesized novel thiohydantoin derivatives and exploration of physicochemical parameters like density, viscosity, ultrasonic velocity, intermolecular free path, adiabatic compressibility etc. The structural elucidation of resultant compounds was done on the basis 1HNMR, IR, Mass etc. The present study revealed that, thiohydantoin analogues shows more structure making capacity in DMSO than DMF.
KEYWORDS:Physicochemical Properties; Refractive Index; 2-Thiohydantoin; Viscosity
Download this article as:| Copy the following to cite this article: Gotmare P. A, Kolhe S. V. Design of Novel Thiohydantoin Derivatives and Exploration their Physico-Chemical Parameters. Orient J Chem 2023;39(5). |
| Copy the following to cite this URL: Gotmare P. A, Kolhe S. V. Design of Novel Thiohydantoin Derivatives and Exploration their Physico-Chemical Parameters. Orient J Chem 2023;39(5). Available from: https://bit.ly/3FooxZC |
Introduction
2-Thiohydantoin is an important class of compounds within chemistry. It is a sulphur derivative of hydantoin which is obtained by replacing the oxygen atom of carbonyl group by sulphur. Thiohydantoin is a intermediate to synthesis of many drugs1-12 .In solid state thiohydantoin shows π-π stacking,hydrogen bonding which is important in pharmaceutical industries13-16
One of the most important things that drew the attention of researchers to synthesized thiohydantoin due to wide range of application like anti-inflammatory, anti-ulcer17, antifungal, antibacterial18, HIV19, hypolipidemic20, antimutagenic21, against HSV22, anticarcinogenic23,on tuberculosis24 and pesticide25 ,derivatives of thiohydantoin are also used as a fungicide26 ,N-phenyl derivative of 2-Thiohydantion shows antiparasitic activity against Trypanosoma brucei species27.
K.H. Chikhalia et al28 reported a series of thiohydantoin derivatives having ethyl linked 3,4-dimethoxyphenylethyl thiourea derivatives with styryl bridge possessing antibacterial properties as well as anti HIV activity. Abubshait S.A.29 synthesized some 2-thiohydantoin drivatives and reported anticancer and antimicrobial properties against gram positive and gram negative bacteria. Kolhe S.V.30 prepared 2-thiohydantoin derivatives by mixing aurones derivative with suitable thiourea by refluxing with KOH and ethaonol as a solvent and reported antimicrobial properties using microbes such as Escherichia coli, Staphylococcus aureus, Klebsilla, Pseudomons. Saied E.M. et al31 synthesized 1, 3-disubstituted 2-thiohydantoin analogues and reported anti-inflammatory activity. Gotmare P.A.et al32 synthesized 2-Thiohydantoin analogous and reported physicochemical properties.
Literature survey reveals that, substituted 2-thiohydantoin were found to be very instrumental in controlling the diseases in the field of medicine, agriculture. The present study has been undertaken to synthesis some new 2-thiohydantoin analogues and test them for their physico-chemical properties.
Materials and Methods
All chemical s and reagents used in this research were commercially sourced and of analytical grade. The purity of resultant compound was check by using TLC. The IR spectra were recorded in KBr by using FT-(IR Perkin Elmer – Spectrum RX-FTIR). Mass spectra were recorded on mass spectrometer while 1HNMR were recorded on FT NMR Spectrometer (Bruker Avance Neo 500 MHz).
General Procedure for synthesis of 2-Thiohydantoin
Aurone (0.01 M) and N-substituted thiourea (0.01 M) were taking in round bottom flask along with 10% KOH and Ethanol as a solvent. A reaction mixture was reflux for 3 h. After this period, the mixture was poured in to ice cold water and filter it by using suction pump. The final product recrystallized with Ethanol.
 |
Scheme 1 Click here to View Scheme |
Table 1
|
Sr. no. |
Compounds |
R1 |
R2 |
R3 |
|
1. |
1a |
C4H3O |
C6H5 |
C6H5 |
|
2. |
1b |
C6H4Cl |
C6H5 |
H |
Preparation of 5-(hydroxyl(4-methoxyphenyl)methyl)-5-(2-hydroxyphenyl)-1,3-diphenyl-2-thioxoimidazolidin-4-one(1a)
2-(4-methoxybenzylidene)benzofuran-3(2H)-one (0.01M) reflux with N,N-diphenyl thiourea (0.01M) in presence of 10% KOH and appropriate ethanol solvent up to 3 hours. After completion of reaction, cooled the mixture and poured in to ice cold water. The solid product obtained which was filter and washed with dilute HCl and water. The product was crystallized by using ethanol.
Mol. Formula C29H24O4N2S: Yellowish Crystalline solid. m.p 258 oC yield 70%, Elemental analysis (%):C,70.14; H,4.87; N,5.64; S,6.46; O,12.89; IR (KBr cm-1) 3617.5 (O-H), 3016 (=CH), 1614 (C=N), 1438 (Ar C=C),ESI-MS[M+H]+ Calculated for C29H24O4N2S: m/z 496.15, 497.15,498.15 ; 1H-NMR (500 MHz, DMSO) δ3.76 (s, 3H), 5.68 (s,1H), 6.86-7.38 (m, J =8.4,1.1 Hz, 11H), 7.43 7.70 (m, 6H),
Preparation of 5-((4-chlorophenyl)(hydroxy)methyl)-5-(2-hydroxyphenyl)-3-phenyl-2-thioxoimidazolidin-4-one (1b)
2-(4-chlorobenzylidene)benzofuran-3(2H)-one(0.01M) reflux with N-phenyl thiourea (0.01M) in presence of 10% KOH and appropriate ethanol solvent up to 3 hours. After completion of reaction, cooled the mixture and poured in to ice cold water. The solid product obtained which was filter and washed with dilute HCl and water. The product was crystallized by using ethanol.
Mol. Formula C22H17O3N2SCl : faint yellowish Crystalline solid, m.p 228oC, yield 74%, Elemental analysis (%):C,62.19; H,4.03; N,6.59; O,11.30; S,7.55;Cl,8.34. IR (KBr cm-1) 3616.5 (O-H), 3268.1 (N-H), 1682(Amide C=O), 1436 (Ar C=C), 755.2 (C-Cl); ESI-MS[M+H]+ Calculated for C22H17O3N2SCl: m/z 424.06, 426.06, 425.07, 427.07. 1H-NMR (500 MHz, DMSO) δ5.58 (s, 1H), 7.04 (m, J = 8.0,7.8 Hz, 1H), 7.48 (m, J = 8.3,1.6,0.5 Hz, 8H), 8.02(m, J = 8.0,1.4 Hz, 1H).
Physicochemical Properties of Thiohydantoin Derivatives
Physico-chemical properties are essential indicators used in hazard, exposure and risk assessments, hence in this experiments the physico-chemical parameters were studied in different solvents, and different concentrations, with temperature 20 °C.
Density and Viscosity
Viscosity and density are affected by temperature. Which implies, for any given fluid, when the temperature is raised, the particle in it start to move apart, bringing down fluid density thereby the value of viscosity also falls down or fluid becomes less viscous. The density and viscosity were taken in different solvent like DMSO and DMF with different concentration and temperature at 20 degree. The density was measured by using pycnometer and viscosity by Ostwald viscometer using fallowing formula.
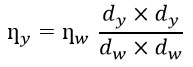
Acoustic parameters
Ultrasonic velocity was useful to determine the strength of material as well as particle interaction in solution hence most of the scientist are attracted toward these parameters. Here ultrasonic parameters was measured using a single-crystal Interferometer (Mittal Enterprises) operating at 1MHz with an accuracy of ±1.0m/s.
The acoustic parameters were determine using fallowing formulae
Adiabatic compressibility (𝛽)
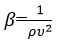
Intermolecular free path length (𝐿𝑓)
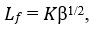
Where 𝐾 is the temperature dependent Jacobson’s constant
Acoustic impedance (𝑍) is given as follows
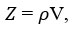
Relative association (RA)
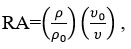
Ultrasonic attenuation (𝛼/𝑓2)
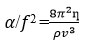
Relaxation time (𝜏)
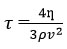
Results and Discussion
The physico-chemical properties of thiohydantoin derivatives were given below
Compound 1a
Table 2: Solvent: DMF Temp. 20 °C
|
Conc. (M) Mol/dm3 |
Density(ρ) Kg/m-3 |
Viscosity(ƞ) ×103 NSm-2 |
Ultasonic velocity(v) m/s |
Refractive Index |
|
0.000 |
970.76 |
0.94577 |
1415.0 |
1.4305 |
|
0.001 |
972.46 |
1.19646 |
1434.4 |
1.422 |
|
0.002 |
972.94 |
1.24280 |
1558.8 |
1.424 |
|
0.003 |
973.88 |
1.30469 |
1603.2 |
1.425 |
|
0.004 |
974.68 |
1.40073 |
1632.0 |
1.426 |
|
0.005 |
976.20 |
1.50860 |
1694.8 |
1.426 |
Table 3: Ultrasonic parameters in DMF
|
Conc. (M) Mol/dm3 |
Adiabetic compressibility (β) |
Intermolecular Free path (Lf) |
Acoustic impedances (Z) |
Relative (RA) |
Ultrasonic Attenuation (∝/ f2 ) x 10-14 |
Relaxation Time (t) |
|
0.000 |
5.14488 |
4.622658 |
1.373625 |
1.000000 |
2.7124 |
6.4869 |
|
0.001 |
4.99790 |
4.556149 |
1.394896 |
0.988202 |
3.28825 |
7.9731 |
|
0.002 |
4.22993 |
4.191512 |
1.516618 |
0.909788 |
2.66007 |
7.0094 |
|
0.003 |
3.99502 |
4.073461 |
1.561324 |
0.885446 |
2.56637 |
6.9498 |
|
0.004 |
3.85210 |
3.999935 |
1.590677 |
0.870535 |
2.60784 |
7.1944 |
|
0.005 |
3.56635 |
3.848718 |
1.654463 |
0.839585 |
2.50397 |
7.1737 |
Table 4: Solvent: DMSO Temp. 20 °C
|
Conc. (M) Mol/dm3 |
Density(ρ) Kg/m-3 |
Viscosity(ƞ) ×103 NSm-2 |
Ultasonic velocity(v) m/s |
Refractive Index |
|
0.000 |
1126.28 |
2.2026 |
1553.0 |
1.4740 |
|
0.001 |
1129.04 |
2.4404 |
1566.2 |
1.4742 |
|
0.002 |
1129.86 |
2.6248 |
1594.6 |
1.4744 |
|
0.003 |
1130.12 |
2.8067 |
1604.0 |
1.4748 |
|
0.004 |
1130.98 |
3.2115 |
1734.2 |
1.4751 |
|
0.005 |
1131.06 |
3.3924 |
1788.2 |
1.4752 |
Table 5: Ultrasonic parameters in DMSO
|
Conc. (M) Mol/dm3 |
Adiabetic compressibility (β) × 10-10 |
Intermolecular Free path (Lf) ×10-11 |
Acoustic impedances (Z) ×106 |
Relative Association (RA)
|
Ultrasonic Attenuation (∝/ f2 ) × 10-14 |
Relaxation Time (t ) ×10-13 |
|
0.000 |
3.68138 |
3.910414 |
1.749110 |
1 |
4.11835 |
10.8115 |
|
0.001 |
3.61074 |
3.872715 |
1.768302 |
0.994001 |
4.43771 |
11.7489 |
|
0.002 |
3.48074 |
3.802360 |
1.801674 |
0.977024 |
4.51924 |
12.1817 |
|
0.003 |
3.43897 |
3.779470 |
1.812872 |
0.971591 |
4.74645 |
12.8695 |
|
0.004 |
2.94000 |
3.494549 |
1.961345 |
0.899266 |
4.29442 |
12.5891 |
|
0.005 |
2.76492 |
3.388900 |
2.022560 |
0.872172 |
4.13954 |
12.5063 |
 |
Figure 1 |
Compound 2a
Table 6: Solvent: DMF Temp. 20 oC
|
Conc. (M) Mol/dm3 |
Density(ρ) Kg/m-3 |
Viscosity(ƞ) ×103 NSm-2 |
Ultasonic velocity(v) m/s |
Refractive Index |
|
0.000 |
970.76 |
0.94577 |
1415 |
1.4305 |
|
0.001 |
971.68 |
1.15997 |
1438.72 |
1.4306 |
|
0.002 |
971.91 |
1.22426 |
1452.81 |
1.4308 |
|
0.003 |
972.18 |
1.30241 |
1464.86 |
1.4309 |
|
0.004 |
972.82 |
1.42826 |
1506.91 |
1.4311 |
|
0.005 |
973.52 |
1.51763 |
1585.68 |
1.4312 |
Table 7: Ultrasonic parameters in DMF
|
Conc. (M) Mol/dm3 |
Adiabetic compressibility (β) × 10-10 |
Intermolecular Free path (Lf) ×10-11 |
Acoustic impedances (Z) ×106 |
Relative (RA)
|
Ultrasonic Attenuation (∝)/f2 × 10-14 |
Relaxation Time (t ) ×10-13 |
|
0.000 |
5.14488 |
4.622658 |
1.373625 |
1.000000 |
2.71240 |
6.4878 |
|
0.001 |
4.97192 |
4.544431 |
1.397975 |
0.984445 |
3.16187 |
7.6897 |
|
0.002 |
4.87479 |
4.499826 |
1.412000 |
0.975128 |
3.24019 |
7.9574 |
|
0.003 |
4.79359 |
4.462190 |
1.424107 |
0.967375 |
3.16000 |
8.3243 |
|
0.004 |
4.52681 |
4.336246 |
1.465952 |
0.941000 |
3.38424 |
8.6206 |
|
0.005 |
4.08530 |
4.119357 |
1.543691 |
0.894898 |
3.08406 |
8.2666 |
Table 8: Solvent: DMSO Temp. 20 oC
|
Conc. (M) Mol/dm3 |
Density(ρ) Kg/m-3 |
Viscosity(ƞ) ×103 NSm-2 |
Ultasonic velocity(v) m/s |
Refractive Index |
|
0.000 |
1126.28 |
2.2026 |
1553.0 |
1.4740 |
|
0.001 |
1127.26 |
2.5128 |
1609.0 |
1.4742 |
|
0.002 |
1127.98 |
2.5247 |
1612.22 |
1.4746 |
|
0.003 |
1128.48 |
2.5382 |
1614.20 |
1.4747 |
|
0.004 |
1128.82 |
2.6141 |
1618.70 |
1.4748 |
|
0.005 |
1129.72 |
2.8663 |
1622.0 |
1.4750 |
Table 9: Ultrasonic parameters in DMSO
|
Conc. (M) Mol/dm3 |
Adiabetic compressibility (β) × 10-10 |
Intermolecular Free path (Lf) ×10-11 |
Acoustic impedances (Z) ×106 |
Relative (RA) |
Ultrasonic Attenuation (∝/f2) × 10-14 |
Relaxation (τ) ×10-13 |
|
0.000 |
3.68138 |
3.910414 |
1.749110 |
1 |
4.11835 |
10.8115 |
|
0.001 |
3.426603 |
3.772675 |
1.813761 |
0.966035 |
4.22099 |
11.4808 |
|
0.002 |
3.410751 |
3.763938 |
1.818551 |
0.964721 |
4.21303 |
11.4817 |
|
0.003 |
3.400881 |
3.758488 |
1.821592 |
0.963965 |
4.21802 |
11.5094 |
|
0.004 |
3.380980 |
3.747475 |
1.827220 |
0.961575 |
4.30687 |
11.7846 |
|
0.005 |
3.645538 |
3.738360 |
1.832405 |
0.960384 |
4.39528 |
12.0510 |
 |
Figure 2 |
Physicochemical properties are a key to determinant of pharmacokinetic and pharmacodynamics profile, and essential to increasing the success rate of drug sample candidates within the preclinical development process. The importance of the physicochemical properties for active transport. The density and viscosity are depends on temperature and concentration, here the density and viscosity increases by increasing concentration but solvent changes change the density and viscosity that means density as well as viscosity affected by solvent.
Ultrasonic velocity in which sound waves travel through liquid sample. Here Ultrasonic velocity increases by increasing concentration due to an increase of cohesive forces which is created due to strong molecular interactions. The experimental Ultrasonic velocity values are different for the same compound in the two different solvents. This suggests that solvent plays an important role in solutions, molecular interactions exists which differs with different solvents. In this case thiohydantoins shows higher Ultrasonic velocity in DMSO solvent than DMF because in DMSO samples shows strong interaction with solvent DMSO.
If intermolecular free path decreases with increase of concentration, explain that the distance between solute and solvent molecules decrease due to increase in solute-solvent interactions, which causes velocity to increase. It is supported by compressibility and relaxation time. Here relaxation time increases by increasing concentration. Compressibility is a measure of the relative volume change of a sample as a response to a pressure change. Here compressibility decreases by increasing concentration that means concentration increases which increase strong interaction between solute and solvent.
Conclusion
It is concluded that physicochemical properties of a thiohydantoin derivatives depends on its structure, concentration and solvents in which it is dissolved. In this case DMSO and DMF shows different values for same compound due to interactions changes in different solvents thereby affecting properties. Further, position of substitution in a compound also affects physicochemical properties. In DMSO solvent, strong solute solvent interaction appear than DMF.
Acknowledgement
We are thankful to Principal Dr. S.H. Pande sir and Department of chemistry Shri Shivaji Arts Commerce and Science College Akot for providing lab equipment’s. Also thankful to Shri D. M. Jakate for his cooperation. We are also thankful to CIL and SAIF, Panjab University, Chandigarh for providing spectral data.
Conflict of Interest
The authors declare no conflict of interest.
References
- Ono, M.; Hayashi, S.; Matsumura, K.; Kimura, H.; Okamoto, Y.; Ihara, M. ; Takahashi, R.; Mori, H.; Saji, H. J.ACS Chem. Neurosci, 2011, 2, 269.
CrossRef - Han, J.; Dong, H.; Xu, Z.; Lei, J.; Wang, M. Int. J. Mol. Sci. 2013, 14, 12484.
CrossRef - Hussain, N.; Joshi, A.; Sharma, C.; Talesara, G.L. Asian J. Chem. 2012, 24, 5917.
- Raghuvanshi, D.S.; Singh K.N. Phosphorus Sulfur Silicon, Rel. Elem. 2010, 185, 2243.
CrossRef - Yao, C.; Zhang, Y.; Zhang, G.; Chen, W.; Yu, Y; Houghten, R. A. Synth. Commun. 2010, 40, 717.
- Carboni, M.; Gomis, J.M.; Loreau, O.; Taran, F. Synthesis. 2008, 40, 417.
CrossRef - Cao, S.; Zhu, L.Z.; Zhao, C.M.; Tang, X.H.; Sun, H.J.; Feng, X.; Qian, X.H., Monatsh. Chem. 2008, 139, 923.
- Sundaram, G.S.M.; Venkatesh, C.; Ila, H.; Junjappa, H. Synlett. 2007, 2 251.
- Wang, Z.D.; Sheikh, S.O.; Zhang, Y. Molecules. 2006, 11, 739.
CrossRef - Reyes, S.; Burgess, K. J. Org. Chem. 2006, 71, 2507.
CrossRef - Porwal, S.; Kumar, R.;Maulik, P.R.; Chauhan, P.M.S. Tetrahedron Lett. 2006, 47, 5863.
CrossRef - Li, J.P.; Ma C.M.; Qu, G.R. Synth. Commun. 2005, 35, 1203.
CrossRef - Bernstein, J. Polymorphism in Molecular Crystals.2002.
- Lu, J.; Rohani, S. Current Medicinal Chem. 2009, 16, 884–905.
CrossRef - Custelcean, R. Chem. Commun. 2008, 295–307.
CrossRef - Jha, S.; Silversides, J.D.; Boyle, R.W.; Archibald, S.J. CrystEng Comm. 2010, 12, 1730-139.
CrossRef - Curran, A. C. W. U. S. Pat.3. 1976, 984,430.
CrossRef - (a) Lacroix, G.; Bascou, J.-P.; Perez, J. Gadras, A. U. S. Pat.6. 2000,018,052. (b) Lacroix, G.;Bascou, J.-P.; Perez, J. Gadras, A. U. S. Pat.5. 1997,650,519. (c) Marton, J.; Enisz, J.; Hosztafi, S.; Timar, T. J. Agric. Food Chem. 1993, 41, 148.
CrossRef - (a) Chérouvrier, J.-R.; Carreaux, F.; Bazureau, J. P. Molecules. 2004, 9, 867.(b) Khodair, A. I.; El-Subbagh, H. I.; El-Emam, A. A. Boll. Chim. Farm. 1997, 136, 561.
CrossRef - (a) Tompkins, J. E. J. Med. Chem. 1986, 29, 855. (b) Elwood, J. C.; Richert, D. A.; Westerfeld, W.W. Biochem. Pharmacol. 1972, 21, 1127.
CrossRef - (a) Takahashi, A.; Matsuoka, H.; Ozawa, Y.; Uda, Y. J. Agric. Food Chem. 1998, 46, 5037. (b)Froelich, E.; Fruehan, A.; Jackman, M.; Kirchner, F. K.; Alexander, E. J.; Archer, S. J. Am. Chem. Soc. 1954, 76, 3099.
CrossRef - El-Barbary, A. A.; Khodair, A. I.; Pedersen, E. B.; Nielsen, C. J. Med. Chem. 1994,37, 73.
CrossRef - Al-Obaid, A. M.; El-Subbagh, H. I.; Khodair, A. I.; Elmazar, M. M. Anticancer Drugs. 1996, 7,873.
- Archer, S.; Unser, M. J.; Froelich, E. J. Am. Chem. Soc. 1956, 78, 6182.
CrossRef - Nagpal, K. L. U. S. Pat.4. 1984,473,393.
- Schroder, L. Eur. Pat. Appl. Ep. 1982, 3, 47.
- Buchynskyy, A.; Gillespie, J.R.; Herbst, Z.M; Ranade, R.M.; Buckner, F.S.; Gelb M.H. ACS Med Chem. Lett. 2017, 8, 886–891
- Patel, R. B., Desai, K.R., Chikhalia, K.H. j. Indian Journal of chem. 2006, 45B,1716-1721.
CrossRef - Abubshait, S.A. j. Indian Journal of chem. 2017, 56B, 641-648.
- Kolhe, S.V. j. I J R B A T. 2017, 5, 101-105.
- Khirallah, S.M.; Ramdan, H.M.; Shawky, A.; Quahl ,S.H.; Baty, R.S.; Alqadri A.; Alsuhaibani, A.M.; Jaremko, M.; Emwas, A.H.; Saied,E.M. j. molecule. 2022, 27,6271.
CrossRef - Gotmare, P. A.; Kolhe, S.V. A Review of the Physicochemical Approach to the Analysis of 2-Thiohydantoin. j. International Journal of Research in Engineering and Science. 2023, 11(9), 134-143.

This work is licensed under a Creative Commons Attribution 4.0 International License.









