Synthesis and Molecular Docking Analysis of New Thiazo-Isoindolinedione Hybrids as Potential Inhibitors of the SARS-Cov-2 Main Protease
Saad Shaaban1,2* , Ahmed A. Al-Karmalawy3
, Ahmed A. Al-Karmalawy3 , Abdulrahman G. Alhamzani4
, Abdulrahman G. Alhamzani4 , Mortaga M. Abou–Krisha4,6
, Mortaga M. Abou–Krisha4,6 , Mahmoud A. Al–Qudah4,5
, Mahmoud A. Al–Qudah4,5 and Tarek A. Yousef4,6
and Tarek A. Yousef4,6
1Department of Chemistry, College of Science, King Faisal University, Al–Ahsa 31982, Saudi Arabia.
2Department of Chemistry, Faculty of Science, Mansoura University, 35516 Mansoura, Egypt.
3Pharmaceutical Chemistry Department, Faculty of Pharmacy, Ahram Canadian University, 6th of October City, Giza 12566, Egypt.
4College of Science, Chemistry Department, Imam Mohammad Ibn Saud Islamic University, Riyadh 11623, Saudi Arabia.
5Department of Chemistry, Faculty of Science, Yarmouk University, P.O. Box 566, Irbid 21163, Jordan.
6Department of Toxic and Narcotic Drug, Forensic Medicine, Mansoura Laboratory, Medicolegal Organization, Ministry of Justice, Egypt.
Corresponding Author E-mail: sibrahim@kfu.edu.sa
DOI : http://dx.doi.org/10.13005/ojc/390412
Article Received on : 06 May 2023
Article Accepted on : 26 Jul 2023
Article Published : 16 Aug 2023
Reviewed by: Dr. G. Dayana Jeyaleela
Second Review by: Dr. Tripti Arora
Final Approval by: Dr. Triveni S
Herein, we report the synthesis of novel thiazo-isoindolinedione derivatives in excellent yields (up to 92%) from the reaction of thiazolidinedione and isoindoline-dione. The structures of the novel compounds were elucidated by 1H-, 13C-NMR, and MS analyses. Furthermore, molecular docking analysis was performed to study the potential inhibition of the SARS-CoV-2 main protease (Mpro) by the new thiazo-isoindolinediones. The present study revealed that the new thiazo-isoindolinediones could inhibit the Mpro and represent a promising platform for the experimental development of new antiviral drugs based on thiazo-isoindolinedione scaffolds.
KEYWORDS:Condensation; COVID-19; Isoindolinedione; Molecular docking; Thiazolidinedione
Download this article as:| Copy the following to cite this article: Shaaban S, Al-Karmalawy A. A, Alhamzani A. R, Abou–Krisha M. M, Al–Qudah M. A, Yousef T. A. Synthesis and Molecular Docking Analysis of New Thiazo-Isoindolinedione Hybrids as Potential Inhibitors of the SARS-Cov-2 Main Protease. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Shaaban S, Al-Karmalawy A. A, Alhamzani A. R, Abou–Krisha M. M, Al–Qudah M. A, Yousef T. A. Synthesis and Molecular Docking Analysis of New Thiazo-Isoindolinedione Hybrids as Potential Inhibitors of the SARS-Cov-2 Main Protease. Orient J Chem 2023;39(4). Available from: https://bit.ly/3OAn1rO |
Introduction
In December 2019, the World Health Organization (WHO) declared COVID-19, a disease caused by the coronavirus 2 (SARS-CoV-2), a global health emergency 1, 2. According to the WHO statistics, SARS-CoV-2 resulted in over 33 million infections and caused more than 1 million deaths 3. There is currently no approved specific treatment for COVID-19; however, immunization can reduce the risk of severe illness and death. The essential chymotrypsin-like cysteine protease (Mpro) is among the potential targets proposed for SARS-CoV-2 inhibition, which plays a crucial role in viral transcription and replication 4-6. Within this context, heterocyclic compounds have been extensively studied as potential lead inhibitors of the SARS-CoV-2 Mpro owing to their diverse biological properties, including antiviral, antiparasitic, and antimicrobial activities, making them among the most investigated pharmacologically active scaffolds 7.
Furthermore, nitrogen- and sulfur-based heterocycles demonstrated favorable binding affinities to various biological targets due to their ability to form exceptionally high intermolecular interactions via the nitrogen and sulfur heteroatoms 8. In this regard, isoindoline-diones serve as a core structure for several medically essential agents. Furthermore, they are commonly utilized as starting building blocks for synthesizing alkaloids, pesticides, and polymers 9. In addition, isoindoline-diones derivatives also manifested potent pharmaceutical properties, such as antiviral, anti-inflammatory, anticancer, and anti-HIV properties 10.
Within this context, isoindolinedione scaffolds were used to synthesize the α-glucosidase inhibitor I 11. Furthermore, isoindoline II is a potent papain-like cysteine protease (PLpro) 11 inhibitor. Moreover, norcantharimide III is a bioactive isoindoledione with potential antitumor activity against breast and lung cancers 12. The 1,3-isoindolinedione tethered triazole IV possessed a promising antituberculosis mycobacterium activity 13.
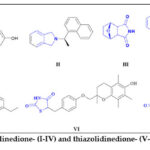 |
Figure 1: Bioactive isoindolinedione- (I-IV) and thiazolidinedione- (V-VI) containing agents. |
Conversely, the thiazolidinedione motif is a common building block of numerous drugs with interesting bioactivity, such as antiviral, antihyperglycemic, antitubercular, and anticancer properties 14-16. The thiazolidinedione-based drug family includes the antidiabetic drugs pioglitazone V, troglitazone VI, and Troglitazone VII 17.
Interestingly, combining bioactive pharmacophores targeting different pathways into a single compound is a major challenge for developing and discovering novel drugs acting simultaneously on multiple targets 18. This strategy has shown considerable success and is currently employed to develop new therapies for diseases such as tuberculosis, malaria, anticancer, and Alzheimer’s diseases 19, 20.
Within this context, we envisage the synthesis of novel thiazo-isoindolinedione hybrids. The synthetic strategy involves a nucleophilic substitution reaction key step between thiazolidine-2,4-dione and bromo-substituted N-alkyl phthalimides. The target compounds are designed to comprise thiazolidine-2,4-dione and 1,3-isoindolinedione linked together by three carbon atoms, as illustrated in Figure 2. Additionally, a molecular docking tool will be used to explore the chemical and electrical properties of the new compounds to inhibit the Mpro required for SARS-CoV-2 replication.
 |
Figure 2: The design criteria of the thiazo-isoindolinedione hybrids. |
Material and Methods
Chemistry
Compounds 2-(2-bromoethyl)isoindoline-1,3-dione (4)21 and 2-(3-bromopropyl)isoindoline-1,3-dione (5) 21, 22 were synthesized from the reaction of 2-bromoethan-1-amine hydrobromide (2) and 3-bromopropylamine hydrobromide (1) with phthalic anhydride under neat conditions at 110 oC, respectively. Furthermore, thiazolidine-2,4-dione (6) 23 was prepared from thiourea and chloroacetic acid reaction using water as the solvent and at 100 oC for 4 hrs. The potassium salt 7 was synthesized by treating an ethanolic solution of thiazolidine-2,4-dione (6) with potassium hydroxideaccording to the reported literature methods24. Copies of the 1H- & 13C-NMR, IR, and MS can be found in the Supporting information.
The synthesis of compound 8
Compound 7 (1.2 mmol) and compound 4 (1 mmol) (1 mmol) were dissolved in DMF (10 ml) and heated at 80 oC for 4 hrs. TLC was used to monitor the process, and after completion, the reaction was poured onto ice to give a white powder.
Compound 8 was obtained from the reaction of compound 4 (1 mmol, 253 mg) with thiazolidine-2,4-dione potassium salt 7 (1.2 mmol, 186 mg) in DMF (10 ml) at 80 oC for 4 hrs. The reaction was followed by TLC (EtOAc/heptane 1:3; Rf = 0.32), isolated as a white solid with 88% yield (and its m.p. = 158–159 oC. 1H NMR (400 MHz, DMSO-d6) δ 7.83 (s, 4H, Ar-H), 4.08 (s, 1H, 2H, CH2S), 3.82 – 3.66 (m, 4H, CH2CH2); 13C NMR (101 MHz, DMSO-d6) δ 172.92, 172.46, 168.21, 134.99, 131.80, 123.59, 40.25, 35.73, 34.21; MS (ESI): m/z = found 327.3 [M++Na]; calcd. 327.0 [M++Na].
The synthesis of compound 9
Compound 9 was obtained from the reaction of compound 7 (1.2 mmol) with compound 5 (1 mmol) in DMF (10 ml) at 80 oC for 4 hrs. The mixture was cooled to room temperature and then poured over ice to give a white powder.
Compound 9 was obtained from compound 5 (1 mmol, 269 mg) and thiazolidine-2,4-dione potassium salt (1.2 mmol, 186 mg) in DMF (10 ml). The reaction was followed by TLC (EtOAc/heptane 1:3; Rf = 0.31), isolated as a white solid with 92% yield and its m.p. = 167–168 oC. 1H NMR (400 MHz, DMSO-d6) δ 7.87 (m, 4H, Ar-H), 4.13 (s, 2H, CH2S), 3.52 (dt, J = 20.3, 7.3 Hz, 4H, 2CH2), 1.90 – 1.77 (m, 2H, CH2); 13C NMR (101 MHz, DMSO-d6) δ 172.75, 172.34, 168.27, 134.84, 132.03, 123.47, 39.38, 35.58, 34.39, 26.42; MS (ESI): m/z = found 376.3 [M+++2Na+K]; calcd. 376.0 [M++2Na+K].
In Silico studies
Molecular docking
The novel two thiazo-isoindolinedione hybrids 8 and 9 were subjected to a molecular docking study using the MOE software 25, 26 to investigate their potential inhibitory effect on the SARS-CoV-2 Mpro. Besides, the co-crystal (O6K) was inserted as a reference standard in the docking process.
Each examined compound was sketched in the ChemDraw and then transferred to the MOE window, subjected to partial charge corrections and energy minimization, as mentioned before 27. Next, the target Mpro protein receptor of SARS-CoV-2 was extracted from the Protein Data Bank (PDB ID: 6Y2G, https://www.rcsb.org/structure/6Y2G) and opened in the MOE window. The Mpro protein was corrected and 3D hydrogenated before energy minimization as a final step of protein preparation 28. Finally, a general docking process was performed by inserting a database of compounds 8 and 9 with the co-crystal (O6K) of SARSCoV-2 Mpro. The default setting options were adjusted to match the selected docking methodology 29.
Notably, a validation process by redocking O6K of SARSCoV-2 Mpro within its receptor pocket was carried out, and the validly applied forcefield was confirmed by obtaining low Root Mean Square Deviation (RMSD) values< 2 Å 30.
Results and Discussion
Synthesis and characterization
Thiazo-isoindolinedione hybrids 8 and 9 were synthesized according to the synthetic scheme 1. The condensation of phthalic anhydride with 2-aminoethyl bromide hydrobromide (2) and 3-aminopropyl bromide hydrobromide (3) under neat conditions afforded compound 4 and compound 5. Furthermore, the reaction of chloroacetic acid and thiourea in water afforded the corresponding 2,4-thiazolidinedione 6. The latter is converted to the corresponding potassium salt via reaction with KOH at room temperature and in ethanol. The nucleophilic substitution reaction of the potassium salt 7 with the bromo derivatives 4 and 5 afforded the corresponding compound 8 and 9 in 88% and 92% yields, respectively.
 |
Scheme 1: The synthesis of thiazo-isoindolinedione hybrids 8 and 9. Reagents: |
In-Silico studies
Molecular docking
The novel two thiazo-isoindolinedione hybrids 8 and 9 were subjected to a molecular docking study to investigate their potential inhibitory effect on the SARS-CoV-2 Mpro. Besides, the co-crystal (O6K) was inserted as a reference standard in the docking process.
Observing the O6K binding mode, it was clear that Glu166 and Cys145 are the most crucial amino acids to produce their inhibitory potential towards the SARS-CoV-2 Mpro. The docked O6K achieved a binding score of -8.41 kcal/mol (RMSD = 1.58 Å) and could bind Glu166, Asn142, and Gly143 with three hydrogen bonds. On the one hand, compound 8 showed a binding score of -5.83 kcal/mol (RMSD = 1.16 Å). It bound crucial amino acids (Glu166 and Cys145) with two pi-hydrogen interactions and one hydrogen bond, respectively. On the other hand, compound 9 interacted with Glu166 (two hydrogen bonds) and Met165 (one pi-hydrogen bond), Table 1. Its binding score was recorded at -6.05 kcal/mol (RMSD = 1.50 Å), superior to compound 8.
Based on the above, compound 9 with the three carbons bridge (propylene) between the 1,3-dioxoisoindoline and thiazolidine-2,4-dione moieties was superior to compound 8 with the two carbons bridge (ethylene) as SARS-CoV-2 Mpro inhibitor. This may be attributed to the flexibility of compound 9, which produced more and deeper fitting within the SARS-CoV-2 Mpro target receptor.
 |
Table 1: 2D interactions, 3D interactions, and 3D positioning of compounds 8 and 9 within the binding pocket of the SARS-CoV-2 Mpro (PDB ID: 6Y2G) target receptor. |
Conclusion
In this study, we designed and synthesized new thiazo-isoindolinedione hybrids from readily available starting materials and in good yields (up to 92%). The chemical structures of the new compounds were characterized by IR, 1H- and 13C-NMR, and MS techniques. In addition, a molecular docking study clarified that compound 9 with the three carbons bridge (propylene) between the 1,3-dioxoisoindoline and thiazolidine-2,4-dione moieties was superior to compound 8 with the two carbons bridge (ethylene) as SARS-CoV-2 Mpro inhibitor. This may be attributed to the flexibility of compound 9, which produced more and deeper fitting within the SARS-CoV-2 Mpro target receptor.
Acknowledgment
This research was supported by the Deanship of Scientific Research, Imam Mohammad Ibn Saud Islamic University (IMSIU), Saudi Arabia, Grant No. (21-13-18-081).
Conflict of Interest
There are no conflict of interest.
Funding Sources
There is no funding sources
References
- Sies, H.; Parnham, M. J., Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radical Biology and Medicine 2020, 156 (20), 107-112
CrossRef - Sanders, J. M.; Monogue, M. L.; Jodlowski, T. Z.; Cutrell, J. B., Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. Jama 2020, 323 (18), 1824-1836.
CrossRef - Li, Q.; Kang, C., Progress in developing inhibitors of SARS-CoV-2 3C-like protease. Microorganisms 2020, 8 (8), 1250.
CrossRef - Houchi, S.; Messasma, Z., Exploring the inhibitory potential of Saussurea costus and Saussurea involucrata phytoconstituents against the Spike glycoprotein receptor binding domain of SARS-CoV-2 Delta (B. 1.617. 2) variant and the main protease (Mpro) as therapeutic candidates, using Molecular docking, DFT, and ADME/Tox studies. Journal of Molecular Structure 2022, 1263, 133032.
CrossRef - Amporndanai, K.; Meng, X.; Shang, W.; Jin, Z.; Rogers, M.; Zhao, Y.; Rao, Z.; Liu, Z.-J.; Yang, H.; Zhang, L., Inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives. Nature communications 2021, 12 (1), 1-7.
CrossRef - Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C., Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582 (7811), 289-293.
CrossRef - Hagar, M.; Ahmed, H. A.; Aljohani, G.; Alhaddad, O. A., Investigation of some antiviral N-heterocycles as COVID 19 drug: molecular docking and DFT calculations. International Journal of Molecular Sciences 2020, 21 (11), 3922.
CrossRef - dos Santos, G. C.; Martins, L. M.; Bregadiolli, B. A.; Moreno, V. F.; da Silva‐Filho, L. C.; da Silva, B. H. S. T., Heterocyclic compounds as antiviral drugs: Synthesis, structure–activity relationship and traditional applications. Journal of Heterocyclic Chemistry 2021, 58 (12), 2226-2260.
CrossRef - Kushwaha, N.; Kaushik, D., Recent advances and future prospects of phthalimide derivatives. Journal of Applied Pharmaceutical Science 2016, 6 (3), 159-171.
CrossRef - Abdel-Hafez, A. A.-M., Synthesis and anticonvulsant evaluation of N-substituted-isoindolinedione derivatives. Archives of pharmacal research 2004, 27, 495-501.
CrossRef - Sherafati, M.; Mohammadi-Khanaposhtani, M.; Moradi, S.; Asgari, M. S.; Najafabadipour, N.; Faramarzi, M. A.; Mahdavi, M.; Biglar, M.; Larijani, B.; Hamedifar, H., Design, synthesis and biological evaluation of novel phthalimide-Schiff base-coumarin hybrids as potent α-glucosidase inhibitors. Chemical Papers 2020, 74, 4379-4388.
CrossRef - Robertson, M. J.; Gordon, C. P.; Gilbert, J.; McCluskey, A.; Sakoff, J. A., Norcantharimide analogues possessing terminal phosphate esters and their anticancer activity. Bioorganic & medicinal chemistry 2011, 19 (18), 5734-5741.
CrossRef - Santos, J. L.; Yamasaki, P. R.; Chin, C. M.; Takashi, C. H.; Pavan, F. R.; Leite, C. Q., Synthesis and in vitro anti Mycobacterium tuberculosis activity of a series of phthalimide derivatives. Bioorganic & medicinal chemistry 2009, 17 (11), 3795-3799.
CrossRef - Hamama, W. S.; Ismail, M. A.; Shaaban, S.; Zoorob, H. H., Progress in the chemistry of 4-thiazolidinones. Journal of Heterocyclic Chemistry 2008, 45 (4), 939-956.
CrossRef - Hamama, W. S.; Ismail, M. A.; Soliman, M.; Shaaban, S.; Zoorob, H. H., Behavior of 2‐Iminothiazolidin‐4‐one with Different Reagents. Journal of Heterocyclic Chemistry 2011, 48 (5), 1169.
CrossRef - Hamama, W. S.; Ismail, M. A.; Shaaban, S.; Zoorob, H. H., Synthesis and biological evaluation of some new Thiazolo [3, 2-a][1, 3, 5] triazine derivatives. Medicinal Chemistry Research 2012, 21 (9), 2615-2623.
CrossRef - Sancheti, P. M.; Pawar, S. P., In vivo toxicity evaluation of troglitazone, rosiglitazone, and pioglitazone in CD-1 Mice. Int J Pharm Biol Sci 2016, 6 (1), 8-15.
- Mansour, M. A.; AboulMagd, A. M.; Abdel-Rahman, H. M., Quinazoline-Schiff base conjugates: in silico study and ADMET predictions as multi-target inhibitors of coronavirus (SARS-CoV-2) proteins. RSC advances 2020, 10 (56), 34033-34045.
CrossRef - Csermely, P.; Agoston, V.; Pongor, S., The efficiency of multi-target drugs: the network approach might help drug design. Trends in pharmacological sciences 2005, 26 (4), 178-182.
CrossRef - Lu, J.-J.; Pan, W.; Hu, Y.-J.; Wang, Y.-T., Multi-target drugs: the trend of drug research and development. PloS one 2012, 7 (6), e40262.
CrossRef - Dato, F. M.; Neudörfl, J.-M.; Guetschow, M.; Goldfuss, B.; Pietsch, M., ω-Quinazolinonylalkyl aryl ureas as reversible inhibitors of monoacylglycerol lipase. Bioorganic chemistry 2020, 94, 103352.
CrossRef - Capela, R.; Magalhaes, J.; Miranda, D.; Machado, M.; Sanches-Vaz, M.; Albuquerque, I. S.; Sharma, M.; Gut, J.; Rosenthal, P. J.; Frade, R., Endoperoxide-8-aminoquinoline hybrids as dual-stage antimalarial agents with enhanced metabolic stability. European Journal of Medicinal Chemistry 2018, 149, 69-78.
CrossRef - Kar, K.; Krithika, U.; Basu, P.; Kumar, S. S.; Reji, A.; Kumar, B. P., Design, synthesis and glucose uptake activity of some novel glitazones. Bioorganic chemistry 2014, 56, 27-33.
CrossRef - Fajkovic, H.; Cha, E. K.; Xylinas, E.; Rink, M.; Pycha, A.; Seitz, C.; Bolenz, C.; Dunning, A.; Novara, G.; Trinh, Q. D.; Karakiewicz, P. I.; Margulis, V.; Raman, J. D.; Walton, T. J.; Baba, S.; Carballido, J.; Otto, W.; Montorsi, F.; Lotan, Y.; Kassouf, W.; Fritsche, H. M.; Bensalah, K.; Zigeuner, R.; Scherr, D. S.; Sonpavde, G.; Roupret, M.; Shariat, S. F., Disease-free survival as a surrogate for overall survival in upper tract urothelial carcinoma. World J Urol 2013, 31 (1), 5-11.
CrossRef - Inc, C., Molecular operating environment (MOE). Chemical Computing Group Inc 2016, 1010.
- Al-Karmalawy, A. A.; El-Gamil, D. S.; El-Shesheny, R.; Sharaky, M.; Alnajjar, R.; Kutkat, O.; Moatasim, Y.; Elagawany, M.; Al-Rashood, S. T.; Binjubair, F. A.; Eldehna, W. M.; Noreddin, A. M.; Zakaria, M. Y., Design and statistical optimisation of emulsomal nanoparticles for improved anti-SARS-CoV-2 activity of N-(5-nitrothiazol-2-yl)-carboxamido candidates: in vitro and in silico studies. Journal of Enzyme Inhibition and Medicinal Chemistry 2023, 38 (1), 2202357.
CrossRef - Ma, C.; Taghour, M. S.; Belal, A.; Mehany, A. B.; Mostafa, N.; Nabeeh, A.; Eissa, I. H.; Al-Karmalawy, A. A., Design and synthesis of new quinoxaline derivatives as potential histone deacetylase inhibitors targeting hepatocellular carcinoma: in silico, in vitro, and SAR studies. Frontiers in chemistry 2021, 22 (9), 725135- 725156.
CrossRef - Khattab, M.; Al-Karmalawy, A. A., Computational repurposing of benzimidazole anthelmintic drugs as potential colchicine binding site inhibitors. Future Medicinal Chemistry 2021, 13 (19), 1623-1638.
CrossRef - Taher, R. F.; Al-Karmalawy, A. A.; Abd El Maksoud, A. I.; Khalil, H.; Hassan, A.; El-Khrisy, E.-D. A.; El-Kashak, W., Two new flavonoids and anticancer activity of Hymenosporum flavum: in vitro and molecular docking studies. J Herbmed Pharmacol 2021, 10 (4), 443-458.
CrossRef - Raslan, M. A.; F. Taher, R.; Al-Karmalawy, A. A.; El-Ebeedy, D.; Metwaly, A. G.; Elkateeb, N. M.; Ghanem, A.; Elghaish, R. A.; Abd El Maksoud, A. I., Cordyline fruticosa (L.) A. Chev. leaves: isolation, HPLC/MS profiling and evaluation of nephroprotective and hepatoprotective activities supported by molecular docking. New Journal of Chemistry 2021, 45 (47), 22216-22233.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









