Role of Alizarin Red-S - NaLS - Ascorbic Acid System in Solar Photogalvanic Performance and Storage
Birama Ram* , Jai Lalita
, Jai Lalita and K.R. Genwa
and K.R. Genwa
Photo-chemistry laboratory, Department of chemistry, Jai Narain Vyas University, Jodhpur-342001, Rajasthan, India.
Corresponding Author E-mail: biramaramjnvujodhpur@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390413
Article Received on : 13 June 2023
Article Accepted on : 16 Aug 2023
Article Published : 22 Aug 2023
Reviewed by: Dr. Sathyanarayanamoorthi venkatachalam
Second Review by: Dr. Mehdi Ahmad
Final Approval by: Dr. Gunawan
The aim of research work was to convert and store the photogalvanic energy by alizarin red-s - NaLS - ascorbic acid. The photogalvanic cells are based on photoelectrochemical nature. This study is better over reductant system i.e., ascorbic acid. The obtained results are comparatively better for sustainable application. M/5000 solution of alizarin red-S, M/1000 solution of NaLS and M/1000 solution of ascorbic acid were used for experimental observation. The fill factor (FF) and conversion efficiency (CE) were calculated for alizarin red-S - NaLS - ascorbic acid system and results were 0.2731 and 1.8970 %, respectively. The electrical outcome was relevant to power, open- circuit voltage, and short circuit current for alizarin red-S - NaLS - ascorbic acid system in solar photogalvanic performance and storage. These results are 197.29 µW, 1075 mV, 672 µA, respectively. The photo-excitation of alizarin red-S molecules was studied in solar performance and storage for photoelectrochemical process.
KEYWORDS:Ascorbic acid; Alizarin red-S; Conversion efficiency; Excitation; Photoelectrochemical; Performance
Download this article as:| Copy the following to cite this article: Ram B, Lalita J, Genwa K. R. Role of Alizarin Red-S - NaLS - Ascorbic Acid System in Solar Photogalvanic Performance and Storage. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Ram B, Lalita J, Genwa K. R. Role of Alizarin Red-S - NaLS - Ascorbic Acid System in Solar Photogalvanic Performance and Storage. Orient J Chem 2023;39(4). Available from: https://bit.ly/3P5O0N3 |
Introduction
Energy is key component biosphere as living standard. Energy is needed for almost anything and everything in life. Consumption of energy is one of the indices in determine the levels of development of a nation. Therefore, availability of crucial for its development. Sunlight is best light source for in alizarin red-S – NaLS – ascorbic acid system in photogalvanic performance and storage. The photogalvanics are photoelectrochemical (PEC) systems based on current flow of the sunlight1,2 (Becquerel,1839). These PEC systems are reported for photo galvanic (PG) effect for sustainability3 (Rideal and Williams,1925).
The photogalvanics are photoelectrochemical properties (PEP) about the iron-thionine component for characteristics about electrochemical nature4-5 (Rabinowitch, 1940). Later on, various researchers6-9 (Clark and Eskert, 1975; Suda, et at., 1978; Fox, et at., 1979; Stevenson and Ervelding, 1981) studied some challenging results for synthesis of technological products.
Later on, Solar energy parameters were discussed for various problems, that’s encountered in the development of the this field10 (Hoffman and Lichtin, 1979). The used of dye reductant with surfactants in PG cells for faster and fairer solar energy transformation and observed the comparative better PG effect11-16 (Ameta et al., 1989; Gangotri and Regar, 1997; Gangotri, et al., 1999; Gangotri and Lal, 2000; Gangotri and Genwa, 2004; Gangotri, and Kumar, 2009, respectively).
Innovative study about PEC system in PG cell were studied by time to time for faster and fairer results. In this order, DSS-tartrazine-EDTA were studied for fairer results17 (Rathore, and Lal, 2018). DSS-Tetrazine-EDTA system in PG cell for was studied for conversion efficiency (CE), t0.5 and fill factor (FF) and obtained results are 0.2800 %, 100.0 minutes and 0.3024, respectively.
Most relevant research work about PG effect in various PEC system was studied for scientific way about directing to dye reductant process18-21 (Genwa and Sonel, 2010; Genwa and Sagar, 2013; Genwa and Singh, 2013; Genwa and Singh, 2017) and some PG cells were developed22-23 (Koli, 2014; Koli and Sharma, 2016).
The natural dye-based surfactant combinations have used for PG cells for ecofriendly environment24 (Rathore et al., 2022). They have studied PG cell for lauryl glucoside-tartrazine- fructose and obtained results in the terms of the CE, t0.5 and FF were recorded as 0.5313%, 100.0 min. and 0.5357, respectively. The PG Cells were also studied at various electrical scale i.e., photocurrent (PC), photopotential (PP), CE, FF and PG cell performance25 (Rathore et al., 2022). They have obtained better results as given: 388.0 µA, 1141.0 mV, 0.7995 %, 0.5389 and 129 minutes in terms of PC, PP, CE, FF and PG cell performance, respectively.
The progressive study about fairer and faster results for prospective energy source through photo-galvanic-system by using of mixed surfactants and subsequently, innovative research work for sustainable development for prospective energy source-based PG cell have been reported. For D-Xylose+MB+Brij-35+NaLS system, CE was observed 0.2812% and PGS performance was found for 120.00 minutes26 (Lal and Gangotri, 2022). The D-Xylose+MB+Brij-35+NaLS PGS for PG cell performance was 110.00 minutes in dark27 (Lal and Gangotri, 2022). The most relevant and recent research work has been reported by innovative study in renewable energy source through mixed surfactant system for eco‑friendly environment28 (Lal and Gangotri, 2023).
Therefore, present study was used in Alizarin red-s Ascorbic acid-sodium lauryl sulphate system as photosensitizer- reductant-anionic surfactant in strong basic medium. Study aims was to investigate the further electrical performance and optimum efficiency of dye- surfactant combination in photogalvanic cell system.
Material and Methods
Materials and Experiments
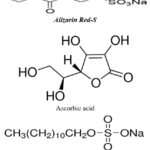
Alizarin red-s dye (Surana scientific Pvt. Ltd., Jodhpur, India) as photosensitizer, Ascorbic acid (Loba Chemie Pvt. Ltd. Mumbai, India) as photo reductant, sodium lauryl sulphate (NaLS), (SISCO Research Laboratories – Mumbai) as surfactant and NaOH (Merck) as basic medium has been used in experimental work. Alizarin red-s dye is M.F. C14H7NaO7S, M.W.- 342.26, Absorption spectra – 530-560 nm, solubility in water, an ethanol and colour-yellow-orange red powder, colour index number: 58005. These solutions were prepared at particular concentration range for faster and fairer results. The used concentrations were 10-3 M,10-5 M,and10-3 M, for Ascorbic acid, Alizarin red-s and sodium lauryl sulphate, respectively. These solutions were prepared by distilled water and store in glass vessels to save them from electromagnetic rays. A mixture of Alizarin Red-S-Ascorbic acid-NaLS and alkaline medium was used in PG cell, Fig.113,15,23,26,27 (Gangotri, et al., 1999; Gangotri and Genwa, 2004; Koli and Sharma, 2016; Lal and Gangotri, 2022).
 |
Scheme 1 Click here to View scheme |
 |
Figure 1: Photogalvanic cell set-up |
Results and Discussion
Effect of variation of Alizarin Red-S (ARS) dye
The concentration of Alizarin Red-S was taken in variable nature, and its concentration ranges from 1.3×10-5 M to 4.0×10-5 M. The comparative better electrical outputs are obtained at 2.7×10-5 M. The alizarin red-S – NaLS – ascorbic acid system in solar photogalvanic performance and storage for potential, current and power were reported in Table 1and Figure 2. Initially, PP and PC increased on increase of Alizarin Red –S dye concentration and obtained the optimum peaks at 2.7×10-5 M, after optimum peaks, on further increase in the concentration of ARS, decrease in the electrical results. At the 1.3×10-5 M (lower concentration) of ARS there are only a few ARS molecules to absorb the major portion of the light and due to this, there was a low electrical output, while at 4.0×10-5 M (higher concentration) of ARS again resulted in decrease in electrical output26,28 (Lal and Gangotri, 2022; 2023).
Table: 1 Concentration of AR-S × 10-5 M with electrical output.
|
Concentration of AR-S × 10-5 M |
PP (mV) |
PC (µA) |
Power (µW) |
|
1.3 |
734 |
438 |
321.49 |
|
2.0 |
862 |
567 |
488.75 |
|
2.7 |
912 |
672 |
612.86 |
|
3.4 |
870 |
574 |
499.38 |
|
4.0 |
728 |
440 |
320.32 |
Effect of variation of Ascorbic acid (AA) reductant
The ascorbic acid was taken in variable nature, and its concentration ranges from 2.04×10-3 M to 2.20×10-3 M. The comparative better results are obtained at 2.12×10-3 M. The alizarin red-S – NaLS – ascorbic acid system in solar photogalvanic performance and storage for potential, current and power were reported in Table 2 and Figure 2. Initially, PP and PC are increased on increase of ascorbic acid concentration and obtained the optimum peaks at 2.12×10-3 M, after optimum peaks, on further increase in the concentration of AA, decrease in the electrical results. Its happed due to presence of ascorbic acid molecules for electron donation to ASR molecules. Higher molarity (2.20×10-3 M) of ascorbic acid resulted in decrease ascorbic acid molecules to reaching surface area of significant path.
Table 2: Concentration of A.A. × 10-3 M with electrical output
|
Concentration of |
PP (mV) |
PC (µA) |
Power (µW)
|
|
2.04 |
725 |
446 |
323.35 |
|
2.08 |
818 |
580 |
474.44 |
|
2.12 |
912 |
672 |
612.86 |
|
2.16 |
824 |
584 |
481.21 |
|
2.20 |
720 |
452 |
325.44 |
Effect of variation of (NaLS) surfactant
The concentration of surfactant was taken in variable nature, and its concentration ranges from 3.2×10-3 M to 4.8×10-3 M. the comparative better results are obtained at 4.0×10-3 M. The alizarin red-S – NaLS – ascorbic acid system in solar photogalvanic performance and storage for potential, current and power were given in Table 3 and Figure 2. Initially, PP and PC both are increased on increase of NaLS concentration and obtained the optimum peaks at 4.7×10-3 M, after optimum peaks, on further increase in the NaLS concentration, electrical output was decreased in the uniform pathway of PG cell27 (Lal and Gangotri, 2022).
Table 3: Concentration of NaLS × 10-3 M with electrical output
|
Concentration of NaLS × 10-3 M |
PP (mV) |
PC (µA) |
Power(µW) |
|
2.8 |
740 |
436 |
322.64 |
|
3.2 |
848 |
568 |
481.66 |
|
3.6 |
912 |
672 |
612.86 |
|
4.0 |
852 |
574 |
489.04 |
|
4.4 |
735 |
430 |
316.05 |
 |
Figure 2: Photopotential, photocurrent and power of PG cell |
Effect of variation of pH
The pH of alizarin red-S – NaLS – ascorbic acid was taken in strong alkaline medium, its ranges from 12.56 to 12.72. the comparative better results are obtained at 12.64. The alizarin red-S – NaLS – ascorbic acid system in solar photogalvanic performance and storage for potential, current and power were reported in Table 4. Initially, PP and PC increased on increase of pH and obtained the optimum peaks, after optimum peaks, on further increase in the pH, electrical output was decreased in the uniform pathway of PG cell24,25 (Rathore et al., 2022).
Table 4: pH with electrical output
|
pH |
PP (mV) |
PC (µA) |
Power (µW) |
|
12.56 |
724 |
445 |
322.18 |
|
12.60 |
852 |
565 |
481.38 |
|
12.64 |
912 |
672 |
612.86 |
|
12.68 |
848 |
560 |
474.88 |
|
12.72 |
730 |
441 |
321.93 |
Characteristics of the PG cell (current – voltage, i-v)
The PG cell containing alizarin red-S – NaLS- ascorbic acid system, the (i-v) characteristics of the cell was significantly reported for charging of the cell18,22,23,26 (Genwa, and Sonel, 2010; Koli, 2014; Koli and Sharma, 2016; Lal and Gangotri, 2022) and the FF was calculated by the following formula11,12,13,15,27 (Ameta et al., 1989; Gangotri and Regar, 1997; Gangotri et al., 1999; Gangotri and Genwa, 2004; Lal and Gangotri, 2022).
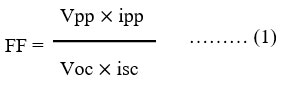
The alizarin red-S – NaLS – ascorbic acid system in solar photogalvanic performance and storage for i-v (current – voltage) characteristics were shown in Table 5 and Figure 3.
Table 5: (I-V) Characteristic table of the Alizarin red-S – NaLS- Ascorbic acid.
|
Potential (mV) |
Current (µA) |
Power (µW) |
FF |
|
1075 |
0 |
0 |
– |
|
1046 |
5 |
5.23 |
– |
|
921 |
20 |
18.42 |
– |
|
890 |
30 |
26.70 |
– |
|
874 |
45 |
39.33 |
– |
|
805 |
96 |
77.28 |
– |
|
784 |
115 |
90.16 |
– |
|
762 |
140 |
106.68 |
– |
|
631 |
285 |
179.83 |
– |
|
615 |
304 |
186.96 |
– |
|
585 |
320 |
187.20 |
– |
|
545 (Vpp) |
362 (ipp) |
197.29 (pp) |
0.2731 |
|
514 |
380 |
195.32 |
– |
|
420 |
441 |
185.22 |
– |
|
395 |
465 |
183.67 |
– |
|
215 |
615 |
132.22 |
– |
|
175 |
634 |
110.95 |
– |
|
98 |
650 |
63.70 |
– |
|
8 |
668 |
5.34 |
– |
|
0 |
672 |
0 |
– |
 |
Figure 3: i-V curve for the PG cell |
Cell performance and conversion efficiency
The alizarin red-S – NaLS – ascorbic acid system in solar photogalvanic performance was obtained in term of half-life periods (t1/2) and its value was 140 minutes. The alizarin red-S – NaLS – ascorbic acid system in solar photogalvanic performance was reported in Table 6. The CE of the PG cell was calculated by the formula11,12,13,15,24,27 (Ameta et al., 1989; Gangotri and Regar, 1997; Gangotri et al., 1999; Gangotri and Genwa, 2004; Rathore et al., 2022, Lal and Gangotri, 2022) and obtained value was 1.8970 %.
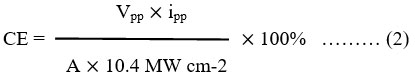
Where Vpp= power point photopotential
Ipp= power point photocurrent
A=electrode area respectively.
Table 6: Study of PG Cell Performance
|
Time (min.) |
Power (µW) |
|
0 |
197.29 |
|
10 |
190.36 |
|
20 |
183.41 |
|
30 |
175.76 |
|
40 |
168.11 |
|
50 |
162.46 |
|
60 |
155.14 |
|
70 |
148.30 |
|
80 |
142.51 |
|
90 |
134.18 |
|
100 |
126.40 |
|
110 |
120.24 |
|
120 |
113.16 |
|
130 |
105.86 |
|
140 (t1/2) |
98.64 (T1/2) |
|
150 |
92.50 |
|
160 |
85.33 |
|
170 |
78.48 |
|
180 |
70.28 |
Mechanism
The alizarin red-S – NaLS – ascorbic acid system in solar photogalvanic performance and storage be may be proposed as follows:
Illuminated chamber
AR-S → AR-S* ……………. (3)
AR –S* + AA → AR–S– + AA+ …………… (4)
At platinum electrode
AR –S– → AR–S + e– …………… (5)
Dark chamber
At counter electrode
AR–S + e– → AR –S– ……………. (6)
AR –S– + AA+ → AR –S + AA ……………… (7)
Where AR–S, AR–S–, AA and AA+ are the Alizarin red-s, its leuco form, Ascorbic acid and its oxidized form, respectively.
Conclusion
The alizarin red-S – NaLS – ascorbic acid system in solar photogalvanic performance and storage be may be proposed for industrial applications. The Alizarin red-s as dye photosensitizer, Ascorbic acid as reductant and NaLS as surfactant combination in PG cell are better for PG cells. PG cells having inbuilt storage capacity and ecofriendly in nature. The low-cost materials are used in these cells. The fairer CE, t0.5 and FF are recorded as 1.8970 %, 140.00 minutes and 0.2731, respectively. The very diluted solution (AR-S × 10-5 M) were used in PG cell, which favour to ecofriendly environment also. Alizarin Red-S-NaLS-Ascorbic Acid photogalvanic cells system shows good prospects for commercially viable.
Acknowledgement
All authors are thankful to Professor (Dr.) Sangeeta Loonker, HOD, Chemistry, JNVU, Jodhpur, India for providing laboratory facility for research work and Birama Ram (Co-author) is thankful to Professor (Dr.) KR Genwa, for research guidance.
Conflicts of Interest
All Authors have no any conflict of interest.
References
- Becquerel, E., Acad. Sci. Paris,1839, 9,14.
- Becquerel, E., Acad. Sci. Paris,1839, 5,61.
- Rideal, E.; Williams, E., J. Chem. Soc., 1925, 127, 258.
CrossRef - Rabinowitch, E., J. Chem. Phy., 1940, 8,551-559.
CrossRef - Rabinowitch, E., J. Chem. Phy., 1940, 8,560-566.
CrossRef - Clark W.; Eskert, J., Solar Energy, 1975, 17,147.
CrossRef - Suda, Y.; Shimaura, Y.; Sakata T.; Tsubomura H., J. Phy Chem., 1978, 82, 268.
CrossRef - Fox, M.; Kabir, U., J. Phys. Chem., 1979, 83,1800.
CrossRef - Stevenson, K.; Ervelding, W., Solar Energy, 1981, 27,139.
CrossRef - Hoffman, M.; Lichtin, N., Solar energy, 1979, 5,153.
CrossRef - Ameta, S.; Khamesra, S.; Chittora A.; Gangotri, KM., Int. J. Energy Res., 1989, 13,643-647.
CrossRef - Gangotri, KM.; Regar, OP., Int. J. Energy Res., 1997, 21,1345.
CrossRef - Gangotri, KM.; Lal C., J. Energy Res., 2000, 42,365.
CrossRef - Rathore, J.; Lal, M., Res. J. Chem. Environ., 2018, 22(6),53-57.
- Genwa, KR.; Sonel, A., J. Indian Chem. Soc.; 2010, 87,685.
- Genwa KR.; Sagar, CP., Int. Phys. Sci., 2013, 8,15-20.
- Genwa KK.; Singh, K., Smart Grid Renew. Energy, 2013, 4,306-311.
CrossRef - Genwa KR.; Singh, SP., Asian Journal of Chem., 2017, 29,12-19.
CrossRef - Koli, P., Applied Solar Energy, 2014, 50(2),67-73.
CrossRef - Koli P., Sharma, U., Applied Solar Energy, 2016, 52,76-83.
CrossRef - Rathore, J.; Arya R.; Sharma, P.; Lal, M., Res. J. Chem. Environ., 2022, 26(6),24-29.
CrossRef - Rathore, J.; Arya, R.; Sharma, P.; Lal, M.; Ind. J. Sci. Tech., 2022, 15(23),1159-1165.
CrossRef - Mohan, L.; Gangotri, KM., Int. J. Energy Res., 2022, 46(14),19538-19547.
CrossRef - Mohan, L.; Gangotri, KM., Journal of Solar Energy Research, 2022, 7(3),1095-1103.
- Lal, M.; Gangotri, K.M., Environ Sci Pollut Res (23 June 2023). (In press) DOI https://doi.org/10.1007/s11356-023-28246-w
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









