Quantification of Recoverable Components of Spent Lithium-Ion Batteries
Amen Kpetemey1* , Sanonka Tchegueni1
, Sanonka Tchegueni1 , Magnoudéwa Bassaï Bodjona1
, Magnoudéwa Bassaï Bodjona1 , Koffi Agbégnigan Degbe1
, Koffi Agbégnigan Degbe1 , Koffi Kili1
, Koffi Kili1 , Gado Tchangbedji1
, Gado Tchangbedji1 , Rachid Idouhli2
, Rachid Idouhli2
1Laboratoire GTVD (Gestion, Traitement et Valorisation des Déchets), Faculté des Sciences, Université de Lomé-Togo.
2Laboratoire Physico-Chimique des Matériaux et Environnement, Faculté des Sciences Semlalia, Université Cadi Ayyad, Marrakech, Maroc.
Corresponding Author E-mail: amenkoffi670@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390414
Article Received on : 01 Jun 2023
Article Accepted on : 11 Aug 2023
Article Published : 16 Aug 2023
Reviewed by: Dr. Aloke Verma
Second Review by: Dr.Subhadra Rajpoot
Final Approval by: Dr. Bashdar Ismael Meena
Recovering spent lithium-ion batteries can help protect the environment and generate added value. The aim of this work is to characterize the various parts of these spent lithium-ion batteries for subsequent recovery of the precious metal elements. The batteries were collected, electrically discharged and dismantled, and the various components quantified. The cathode powder obtained after basic leaching was characterized by ICP and XRD. The batteries consist of steel (21.10%) and plastic shells, the anode (24.40%), the electrolyte-soaked separator and the cathode (35.86%). The anode consists of graphite deposited on a copper foil representing 15.15% of its weight, and the cathode of aluminum foil (3.93%) and lithium cobalt oxide. Physico-chemical characterization of the cathode powder yielded CoO (65.30%), Li2O (5.39%), MnO (15.78%) and NiO (2.17%). At the end of this study, we note the presence of precious metals, on which our subsequent recovery work will focus.
KEYWORDS:Anode; Cathode; Environment; Lithium-Ion Battery; Metals
Download this article as:| Copy the following to cite this article: Kpetemey A, Tchegueni S, Bodjona M. B, Degbe K. A, Kili K, Tchangbedji G, Idouhli R. Quantification of Recoverable Components of Spent Lithium-Ion Batteries. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Kpetemey A, Tchegueni S, Bodjona M. B, Degbe K. A, Kili K, Tchangbedji G, Idouhli R. Quantification of Recoverable Components of Spent Lithium-Ion Batteries. Orient J Chem 2023;39(4). Available from: https://bit.ly/3E0aCbM |
Introduction
The development of the electronics industry to improve the living conditions of man on earth. This development generates the waste known as waste electrical and electronic equipment (WEEE) which is a global problem 1. In 2019, the world generated an impressive 53.6 Mt of electronic waste, an average of 7.3 kg per capita. This generated waste contains toxic or hazardous materials but also rare or precious metals 2. The regions most affected by WEEE exports are Southeast Asia and West Africa. Togo, like the African countries, is affected by its second-hand WEEE products which end up in garbage cans, on landfills and sometimes even incinerated. Since there is no appropriate sector dedicated to the management of WEEE waste. Batteries, printed circuit boards (PCBs), liquid crystal displays (LCDs), cathode ray tubes (CRTs), hard disk drives (HDDs), refrigerators and mobile phones are an integral part of a typical WEEE 3 .
Lithium-ion batteries (LIB), one of the electrical and electronic equipment, has been growing rapidly due to the prevailing implementation of new technologies in electronics, consumer attractive designs and demands of life daily. These batteries are widely used in mobile phones, laptops, video cameras due to their characteristics of light weight, high energy and good performance 4,5. The lifetime of these batteries generally varies from 2 to 4 years 6,7. The strong growth in LIB production due to the proliferation of portable equipment generates huge quantities of spent lithium-ion batteries, the management of which constitutes an environmental problem.
Global demand for metals is increasing. However, the exploitable reserve, which is only distributed in a few countries, is very limited and is running out 8. In other words, the mismanagement of LIB leads to a waste of metal resources and environmental risks 9.
If spent LIBs are simply disposed of, a serious environmental problem would be caused by the leakage of organic electrolytes as well as a risk of explosion or fire due to the lithium it contains.
A lithium-ion battery consists of a cathode, a separator and an anode, all enclosed in a steel or plastic casing. The cathode has been made of lithium cobalt oxide (LiCoO2), since its first synthesis by Goodenough in 1980 10 and its first commercialization by Sony in 1991 11. Subsequently, various Li-containing materials, such as LiFePO4 12, LiMn2O4 13, and LiNi x MnyCoz O2 14, were developed and widely applied. However, LiCoO2 is often used in mobile phone batteries due to its competitiveness and energy density 15. The anode is a thin sheet of copper coated with graphite.
When lithium-ion battery waste is properly treated, precious metals such as cobalt and lithium can be recovered. From the point of view of preserving the environment and recovering valuable resources, the recycling of spent lithium-ion batteries is highly desirable. The current state of the recycling process has been examined in several studies 16,17,18.
The precious metal elements (Co, Li, Mn, Ni, Al, Cu, etc.) contained in the battery electrodes are essential. Lithium is an indispensable element in the manufacture of electrode materials for batteries and in other areas such as glass ceramics, enamels, adhesives, lubricating greases, metal alloys, air conditioning and dyeing 19. Cobalt powders have been used in steel for cutting tools, in composites reinforced by abrasion, and in alkaline rechargeable batteries 20. Additionally, recycling LIB batteries preserves primary resources for the future by recovering the precious metals associated with LIB batteries. In addition, LIB recycling is attractive for commercial production because it is free of siliceous matrix and its high concentration of metal ions compared to primary sources. It is therefore very important to recover these metallic elements for economic, health and environmental reasons.
It is in this logic that for decades, research on the recovery of precious metallic elements contained in LIB waste has been carried out. The main recycling technologies used are pyrometallurgy, biometallurgy and hydrometallurgy.
This work was undertaken on spent lithium-ion batteries from mobile phones. This involves collecting, discharging and dismantling spent lithium-ion batteries in order to recover and quantify the various components of the battery. Then the cathodic powder obtained after basic leaching with soda (NaOH) was characterized by ICP and XRD, thus revealing the chemical composition and the different phases present, with their content.
Methodology
Collection and sorting of spent batteries
Spent Lithium-ion cell phone batteries were collected from second-hand cell phone dealers in the Lomé port area and from repairers. The potential difference of all the batteries used in this study was 3.7 V. The batteries collected were of different brands, wattages, sizes and origins. They were sorted and grouped according to brand and power. They include brands such as Samsung, HTC, SonyEricson, Nokia…
 |
Figure 1: Spent lithium-ion batteries |
Dismantling of lithium-ion batteries
For safety reasons, prior to disassembly, the spent lithium-ion batteries were soaked in a 10% NaCl solution for 24 hours to discharge them, in order to avoid any danger of short-circuiting or auto-ignition.
The batteries were then dismantled manually, using pliers and saws. The steel casings were cut and removed. The internal components were unrolled, separating the anodes, cathodes and plastic separators.
 |
Figure 2: Components of the spent lithium-ion battery |
Quantification of the different components recovered from the lithium-ion batteries
The mass of each component (steel, paper, plastic, electrodes, etc.) of spent lithium-ion batteries was determined using a Nimbus brand balance.
 |
Figure 3: Nimbus Scale |
The content of the various components obtained was evaluated using the formula:
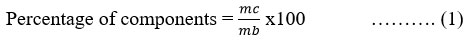
mc: mass of the component recovered from the battery
mb : mass of the lithium-ion battery
Separation of copper at the anode
Once the anode had been dismantled, it was immersed in demineralized water in a beaker and stirred for 2 hours. The graphite detached from the copper foil, allowing recovery of the copper in its metallic form, which was dried and weighed. The mass thus obtained was used to calculate the copper content of the anode using the following formula:
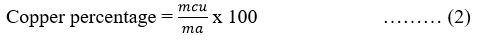
mcu: mass of copper obtained
ma: mass of the anode
Preparation and characterization of cathode powder
The cathode was treated with a known volume of 1M NaOH solution (1:5) for 2 hours with magnetic stirring to dissolve the aluminum foil which serves as an electron collector. The cathode material separated from the aluminum was recovered after filtration and washing to constant pH, in order to eliminate the residual sodium hydroxide before being dried in an oven at 105°C for 24 hours and ground to 75 μm . Then the powder is kept for leaching studies and analyses.
Aqua regia (HCl: HNO3, ratio 3:1) was used for the complete digestion of the LIB cathode powder before the quantification of the metal contents.
The determination of the metallic elements contained in the cathode powder was carried out using an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES), brand (Thermo Fisher X Series II, Thermo Fisher Scientific , Bremen, Germany) after digestion with Lugol water (HNO3 :HCl=1:3, v/v)
Crystal phases of the raw cathode were determined by X-ray diffraction, using a Rigaku Miniflex 300 instrument operating in the 2θ 5-98° range, with Cu Kα radiation (λ = 0.15418 nm), a voltage of 45kv, and a current of 40 mA.
Results and Discussion
Quantification of the different parts of the lithium-ion battery
The results of the quantification of the various parts of the lithium-ion batteries studied are recorded in table 1. The average mass of the batteries studied is 25.5 g. These results reveal that the cathode and the anode represent an important part of the lithium-ion battery since they concentrate more than half (60%) of the mass of the batteries. This is in agreement with the work of 21,22. These two components constitute the active part of lithium-ion batteries and contain recoverable metallic elements (aluminum, copper, cobalt, nickel, lithium and manganese, etc.). The steel boxes, representing 21% of the batteries by mass, protect the active components of the batteries. Other materials (plastics, paper and separators) account for 11% of battery mass 23.
Table 1: Proportion of the different components of the batteries studied
|
Battery weight (g) |
% Anode |
% Cathode |
% Steel |
% Others |
|
25,49±7,41 |
24,40±7,84 |
35,86±7,08 |
21,10±1,36 |
11,67±3,31 |
The steel (21% of the battery) and the plastic material obtained following dismantling can directly return to the recycling circuit. The anode and the cathode can undergo treatments with a view to recovering the metallic elements which they contain. 124,25.
Pre-separation of battery components would be very cost effective for recycling, since the steel case, paper, plastic, separator, aluminum and copper foils, solvent are all directly recyclable after separation 26.
Characterization of the electrodes
Table 2, shown below, summarizes the results of the contents of metallic elements (Cu and Al) and of the cathodic and anodic materials. The anode, having an average mass of 6.22g, consists of a copper film whose proportion is evaluated at 15.15%. The cathode with an average mass of 9.14g consists of an aluminum film (3.93%). This aluminum film is connected to an active cathodic powder thanks to a binder, the whole of which has a content of 96.07% and contains metal oxides. These results differ from those found by 27,28,29, who respectively found for copper a content of 9%; 5.5% for aluminum and 8.84% for copper and aluminum together. As pointed out
Georgi-Maschler et al. (2012) 28, battery producers produce their own specific types of LIBs, so it is difficult to provide precise general values. It is therefore difficult to provide precise general values for the masses of the electrode components of a lithium-ion battery, since the composition varies depending on the manufacturing process 29.
Table 2: Proportion of copper and aluminum in lithium-ion battery electrodes
|
Anode |
% Components |
Cathode |
%Components |
|
Copper |
15,15 |
aluminum |
3,93 |
|
Graphite + binders |
84,85 |
Cathodic powder + binders |
96,07 |
Copper and aluminum can be recovered in the metallic state by hydrometallurgical or pyrometallurgical and biometallurgical means
Characterization of the cathode powder
Elementary chemical analysis at the ICP and mineralogical analysis by X-ray diffraction made it possible to characterize the cathode powder. The results of its analyzes are recorded in Table 3 and Figure 4. The ICP analysis showed that the cathodes of the batteries studied are essentially made up of Cobalt (CoO, 65.30%), Manganese (MnO 15 .78%), lithium (Li 2 O 5.39%) and traces of nickel (NiO 2.17%). These results show that the cathode powder is rich in cobalt oxide, and a minor phase of manganese oxide, which is used in some cathodes to reduce battery production costs, since cobalt is expensive 30-36. Consequently, given the high content of Mn in the sample, we will think about its extraction, as well as for Co and Li.The nickel content is thought to be due to doping and surface modification used to increase battery capacity. 37,38.
Table 3: Content of metallic ingredients in the cathode of Lib
|
|
%CoO |
%MnO |
%Li2O |
%NiO |
|
Cathode |
65,30 ±1,09 |
15,78±1,15 |
5,39±0,42 |
2,17±0,50 |
The X-ray diffraction analysis, carried out to identify the phases present in the cathode powder, showed the presence of three crystalline phases. Lithium cobalt oxide LiCoO2 (18.9°, 37.32°, 39°, 45°, 49°, 59°, 65°, 69°) constitutes the major phase of the material with a proportion of 68.4%. The manganese and lithium oxide LiMn2O4 (36.32°, 44°, 58.29°, 63.99°) is also present with a proportion of 31.6%.
We note a third minority phase whose peak is detectable at 2θ = 26.6° corresponds to graphite.
 |
Figure 4: X-ray diffraction analysis of the cathode electrode of the lithium-ion battery. |
The presence of this third phase shows that the cathode contains some graphite 37,39,40
The cathode powder obtained is a mixture of cathodes from batteries of different brands and origins. It is therefore possible to have cathodes composed of LiCoO2, of LiMn2O4 or a combination of the two oxides. But we can remember that the cathodes are mainly composed of LiCoO2.
The presence of nickel in the cathode was not detected by X-ray diffraction, although chemical analysis revealed a NiO content of 2%. This can be explained by the fact that nickel was used to dope LiCoO2 and LiMnO2. 37,38
From the cathodes of spent lithium-ion batteries of mobile phones, lithium, cobalt, manganese and nickel can be recovered. The recovery of its useful metals from spent lithium-ion batteries is an asset for the environment since the extraction of these metals has enormous impacts on the environment.
To recover these metals from the cathode, the techniques of leaching and sequential precipitation 41-43, electrolysis 44-46 or solvent extraction. 47,48,49
Conclusion
At the end of this work, we note that spent lithium-ion batteries contain recoverable components. The characterization of these batteries shows that steel, plastic, aluminum and copper can be recovered using simple techniques. The constitution of cathode powder, the active material of lithium-ion batteries, reveals that it consists of lithium and cobalt oxide, lithium manganese oxide and nickel oxide. These metals can be recovered after leaching by precipitation, electrolysis or solvent extraction.
In order to minimize the environmental impacts of the recovery of these useful metals, it is important to develop environmentally friendly leaching and recovery techniques.
Acknowledgement
The authors warmly thank the Physico-Chemical Laboratory of Materials and Environment of the Semlalia Faculty of Sciences (Cadi Ayyad University, Marrakech-Morocco), the Laboratory of the General Directorate of Mines and Geology (LDGMG) and the Laboratory of Analysis of Environmental Geochemistry (LAGE) from the University of Lomé for having accepted to open their doors to our various requests for analyzes carried out.
Conflict of Interest
The authors declare that there is no conflict of interest to be reported.
Funding Sources
There are no funding sources.
References
- Yao, L.; He, W.; Li, G.; and Huang, J. The integrated design and optimization of a WEEE collection network in Shanghai, China. Waste Management & Research., 2013,31,910-919. https://doi.org/10.1177/0734242X13487583
CrossRef - Forti V.; Baldé C.P.; Kuehr R.; Bel G. The Global E-waste Monitor: Quantities, flows and the circular economy potential. UNU/UNITAR and ITU., 2020,2,13-120.
- Zhang, L.; Xu, Z. A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. Journal of Cleaner Production., 2016,127, 19-36. https://doi.org/10.1016/j.jclepro.2016.04.004.
CrossRef - Lundblad, A.; Bergman, B. Synthesis of LiCoO2 starting from carbonate precursors. Solid State Ionics., 1997, 96, 183-193. https://doi.org/10.1016/S0167-2738(97)00016-7
CrossRef - Plichta, E.; Salomon, M. Rechargeable Li/LixCoO2 cell. J. Power Sources., 1987, 21, 25-31 https://doi.org/10.1016/0378-7753(87)80074-5
CrossRef - Wang, W.; Zhang, Y.; Liu, X.; and Xu, S. A Simplified Process for Recovery of Li and Co from Spent LiCoO2 Cathode Using Al Foil As the in Situ Reductant. ACS Sustainable Chem.Eng., 2019, 7, 12222–12230. https://doi.org/10.1021/acssuschemeng.9b01564
CrossRef - Yang, Y.; Lei, S.; Song, S.; Sun, W.; and Wang, L. Photocatalytic properties of Co3O4 /LiCoO2 recycled from spent lithium-ion batteries using citric acid as leaching agent. Waste Manage.,2020, 102, 131–138. https://doi.org/10.1016/j.matchemphys.2017.01.003
CrossRef - Xu, J.; Wang, X.; Yuan, N.; Hu, B.; Ding, J.; Ge, S. Graphite-based lithium ion battery with ultrafast charging and discharging and excellent low temperature performance. J. Power Sources.,2019, 430, 74–79. https://doi.org/10.1016/j.ensm.2019.04.033
CrossRef - Kang, D H P.; Chen, M.; Ogunseitan, O. A. Potential environmental and human health impacts of rechargeable lithium batteries in electronic waste. Environ. Sci. Technol., 2013, 47, 5495−5503. https://doi.org/10.1021/es400614y
CrossRef - Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0<x≤1): A new cathode material for batteries of high energy density. Mat. Res. Bull., 1980, 15, 783−789. DOI:10.1016/0025-5408(80)90012-4
CrossRef - Xiao, J.; Li, J.; Xu, Z. Challenges to Future Development of Spent Lithium Ion Batteries Recovery from Environmental and Technological Perspectives. Environ. Sci. Technol., 2020, 54, 9−25. DOI: 10.1021/acs.est.9b03725
CrossRef - Zhang, X.; Hulzen, M. V.; Singh, D. P.; Brownrigg, A.; Wright, J. P.; Dijk, N. H.; Wagemaker, M. Direct view on the phase evolution in individual LiFePO4 nanoparticles during Li-ion battery cycling. Nat. Commun., 2015, 6,83-33. DOI: 10.1038/ncomms9333
CrossRef - Xiao, J.; Li, J.; Xu, Z. Novel approach for in situ recovery of lithium carbonate from spent lithium ion batteries using vacuum metallurgy. Environ. Sci Technol., 2017, 51, 11960−11966. https://doi.org/10.1016/j.jhazmat.2017.05.024
CrossRef - Zheng, J.; Engelhard, M. H.; Mei, D.; Jiao, S.; Polzin, B. J.; Zhang, J. G.; Xu, W. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy., 2017, 2, 17012. https://doi.org/10.1038/nenergy.2017.12
CrossRef - Yoon, M.; Dong, Y.; Yoo, Y.; Myeong, S.; Hwang, J.; Kim, J.; Choi, S. H.; Sung, J.; Kang, S. J.; Li, J.; Cho, J. Unveiling nickel chemistry in stabilizing high-voltage cobalt-rich cathodes for lithium-ion batteries. Adv. Funct.Mater., 2020, 190-7903. DOI:10.1002/adfm.201907903
CrossRef - Bernardes, A.M.; Espinosa, D.C.R.; Tenorio, J.A.S. Collection and recycling of portable batteries: a worldwide overview compared to the Brazilian situation. J. Power Sources., 2003, 124, 586–592. DOI:10.1016/S0378-7753(03)00810-3
CrossRef - Bernardes, A.M.; Espinosa, D.C.R.; Tenorio, J.A.S. Recycling of batteries: a review of current processes and technologies. J. Power Sources.,2004, 130, 291– 298. https://doi.org/10.1016/j.jpowsour.2003.12.026
CrossRef - Espinosa, D.C.R.; Bernardes, A.M.; Tenorio, J.A.S. An overview on the current processes for the recycling of batteries. J. Power Sources., 2004, 135, 311 –319. https://doi.org/10.1038/nenergy.2017.12
CrossRef - Nguyen, T.H.; and Lee, M.S. A review on the separation of lithium ion from leach liquors of primary and secondary resources by solvent extraction with commercial extractants. Processes., 2018, 6, 1–15. https://doi.org/10.3390/pr6050055
CrossRef - Nguyen, T.H.; Lee, M.S. Development of a hydrometallurgical process for the recovery of calcium molybdate and cobalt oxalate powders from spent hydrodesulphurization (HDS) catalyst. Journal of Cleaner Production., 2015, 90, 388–396. https://doi.org/10.1016/j.jclepro.2014.11.048
CrossRef - Shuva, M.A.H.; Kurny, A.S.W. Hydrometallurgical recovery of value metals from spent lithium ion batteries, Am. J. Mater. Eng. Technol., 2013, 1,8–12. DOI:10.12691/materials-1-1-2
- Gamiño-Arroyo, Z.; Quintero-Almanza, D.; Sánchez-Cadena, L.E.; Gómez-Castro, F.I.; Uribe-Ramírez, A.R.; Aguilera-Alvarado, A.F.; and Ocampo Carmona, L.M. Recovery of Cobalt from Spent Lithium-Ion Mobile Phone Batteries Using Liquid–Liquid Extraction Journal of Batteries., 2019, 5, 44 https://doi.org/10.3390/batteries5020044
CrossRef - Horeh, N.B.; Mousavi, S.; Shojaosadati, S. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. J. Power Sources., 2016, 320, 257–266. https://doi.org/10.1016/j.jpowsour.2004.03.083
CrossRef - He, Y.; Li, J.; Fu, Y.; Xie, W.; Feng, Y.; Alejandro, K. Hydrometallurgical enhanced liberation and recovery of anode material from spent lithium-ion batteries. Waste Management.,2021, 126, 517–526. https://doi.org/10.1016/j.wasman.2021.03.052
CrossRef - Bajaj, H.C.; Natarajan, S.; Boricha, A.B. Recovery of value-added products from cathode and anode material of spent lithium-ion batteries. Waste Management.,2018,77,455-465. https://doi.org/10.1016/j.hydromet.2009.08.005
CrossRef - Paulino, J.F.; Busnardo, N.G.; Afonso, J.C. Recovery of valuable elements from spent Li-batteries. Journal of Hazardous Materials., 2008, 150, 843–849. https://doi.org/10.1016/j.jhazmat.2007.10.048
CrossRef - Xu, J.Q.; Thomas, H.R.; Francis, R.W.; Lumb, K.R.; Wang, J.; Liang, B. A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources., 2008, 177, 512-527. https://doi.org/10.1016/j.jpowsour.2016.04.104
CrossRef - Georgi-Maschler, T.; Friedricha, B.; Weyheb, R.; Heegnc, H.; Rutzc, M. Development of a recycling process for Li-ion batteries. Journal of Power Sources., 2012, 207, 173– 182. https://doi.org/10.1016/j.jpowsour.2007.11.074
CrossRef - Bertuol, D.A.; Machado, C.M.; Silva, M.L.; Calgaro, C.O.; Dotto, G.L.; Tanabe, E.H. Recovery of cobalt from spent lithium-ion batteries using supercritical carbon dioxide extraction. Waste Management.,2016, 51,245-251. https://doi.org/10.1016/j.wasman.2016.03.009
CrossRef - Boer, M.A.; and Lammertsma, K. Scarcity of Rare Earth Elements. ChemSusChem.,2013,6, 2045-2055. DOI: 10.1002/cssc.201200794
CrossRef - Swart, P.; Dewulf, J.; Biernaux, A. Resource demand for the production of different cathode materials for lithium ion batteries. Journal of Cleaner Production.,2014,84, 391-399
CrossRef - Huang, Y.; Han, G.; Liu, J.; Chai, W.; Wang, W.; Yang, S.; Su, S. A stepwise recovery of metals from hybrid cathodes of spent Li-ion batteries with leaching-flotation-precipitation process. Journal of Power Sources.,2016,325, 555-564. https://doi.org/10.1016/j.jpowsour.2016.06.072
CrossRef - Xin, Y.; Guo, X.; Chen, S.; Wang, J.; Wu, F.; Xin, B. Bioleaching of valuable metals Li, Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery. Journal of Cleaner Production.,2016,116,249-258. https://doi.org/10.1016/j.jclepro.2016.01.001
CrossRef - Xiao, J.; Li, J.; Xu, Z. Recycling Metals from Lithium Ion Battery by Mechanical Separation and Vacuum Metallurgy. Journal of Cleaner Production.,2017,338,124-131. DOI : 10.1016/j.jhazmat.2017.05.024
CrossRef - Jhy-Chern, L.; Lie, J.; and Tanda., S. Subcritical Water Extraction of Valuable Metals from Spent Lithium-Ion Batteries. Journal of Molecules., 2020, 25, 21–66.https://doi.org/10.3390/molecules25092166
CrossRef - Anwani, S.; Methekar, R.; and Ramadesigan, V. Resynthesizing of lithium cobalt oxide from spent lithium-ion batteries using an environmentally benign and economically viable recycling process. Hydrometallurgy., 2020, 197, 105-430 https://doi.org/10.1016/ j.hydromet.105430.
CrossRef - Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic acid-based leaching system: A sustainable process for recovery of valuable metals from spent Li-ion batteries. Journal of Power Sources.,2015, 282, 544-551. https://doi.org/10.1016/j.jpowsour.2015.02.073
CrossRef - Chen, L.; Tang, X.; Zhang, Y.; Li, L.; Zeng, Z.; Zhang, Y. Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Journal of Hydrometallurgy., 2011,108, 80–86. https://doi.org/10.1016/j.hydromet.2011.02.010
CrossRef - Xu, M.; Kang, S.; Jiang, F.; Yan, X.; Zhu, Z.; Zhao, Q.; Tenga Y.; and Wanga, Yu. A process of leaching recovery for cobalt and lithium from spent lithium-ion batteries by citric acid and salicylic acid. Journal of RSC Adv., 2021,11, 27-689. DOI https://doi.org/10.1039/D1RA04979H
CrossRef - Li, L.; Lu, J.; Ren, Y.; Zhang, X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. Journal of Power Sources., 2012, 218, 21-27. https://doi.org/10.1016/j.jpowsour.2012.06.068
CrossRef - Chen, X.; Kang, D.; Cao, L.; Li, J.; Zhou, T.; and Ma, H. Development of a metal recovery process from Li-ion battery wastes. Purif Technol., 2009, 210, 690–697. https://doi.org/10.1016/j.hydromet.2005.06.004
CrossRef - Wang, R-C.; Lin, Y.-C.; and Wu, S.-H. A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy., 2009, 99, 194–201. https://doi.org/10.1016/j.hydromet.2009.08.005
CrossRef - Barik, S.; Prabaharan, G.; and Kumar, B. An innovative approach to recover the metal values from spent lithium-ion batteries Waste Management, 2016, 51, 222–226. https://doi.org/10.1016/j.wasman.2015.11.004
CrossRef - Pranolo, Y.; Zhang, W.; & Cheng, C. Recovery of metals from spent lithium-ion battery leach solutions with mixed solvent extractant system. Hydrometallurgy.,2010,102,37-42. https://doi.org/10.1016/j.wasman.2015.11.004
CrossRef - Lemaire, J. ; Svecova, L. ; Lagallarde, F. ; Laucournet, R. ; Thivel, P-X. Lithium recovery from aqueous solution by sorption/desorption. Hydrometallurgy.,2014, 143,1-11. https://doi.org/10.1016/j.hydromet.2010.01.007
CrossRef - Li, Q.; Fung, K.Y.; Ng, K.M. Hydrometallurgy process for the recovery of valuable metals from LiNi0,8Co0,15Al10,05O2 cathode materials. SN Applied Sciences., 2019, 1, 6-90 https://doi.org/10.1007/s42452-019-0705-z
CrossRef - Wang, F.; Sun, R.; Xu, J.; Chen, Z.; and Kang, M. Recovery of cobalt from spent lithium ion batteries using sulphuric acid leaching followed by solid-liquid separation and solvent extraction. RSC Adv.,2016, 6, 85303–85311. DOI: 10.1039/C6RA16801A
CrossRef - Jha, A.K.; Jha, M.K.; Kumari, A.; Sahu, S.K.; Kumar, V.; and Pandey, B.D. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Purif. Technol., 2013, 104, 160–166. DOI https://doi.org/10.1039/C6RA16801A
CrossRef - Suzuki, T.; Nakamura, T.; Inoue, Y.; Niinae, M.; and Shibata. J. hydrometallurgical process for the separation of aluminum, cobalt, copper and lithium in acidic sulfate media Purif. Technol., 2012, 98, 396–401. https://doi.org/10.1016/j.seppur.2012.06.034
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









