Acoustical and Spectroscopical Analysis of Surfactant with Different Alcohols at Various Concentrations and Temperatures
S. Aruna1* , R. Thiyagarajan2
, R. Thiyagarajan2 , A. Panneerselvam3
, A. Panneerselvam3 and S. Gnanasaravanan4
and S. Gnanasaravanan4
1Department of Physics, Bharathiyar University, Coimbatore (Tamil Nadu) India.
2Department of Physics, Chikkaiah Naicker College, Erode (Affiliated to Bharathiyar University, Tamil Nadu) India.
3Department of Physics, Vivekanandha College of Engineering for Women (Autonomous), (Affiliated to Anna University, Chennai), Elayampalayam, Tiruchengode, (Tamil Nadu) India.
4Khadir Mohideen College (Affiliated to Bharathidasan University, Thiruchirappalli), Adirampattinam, Thanjavur, Tamil Nadu, India.
Corresponding Author E-mail: shastha.saruna5@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390417
Article Received on : 08 Jul 2023
Article Accepted on : 18 Aug 2023
Article Published : 29 Aug 2023
Reviewed by: Dr. Aisha Mahmood A. Turkustani
Second Review by: Dr. Selvaraju K
Final Approval by: Dr. Guna Shekar
The ultrasonic velocity of molecules in medium supplies valid information about the binding forces between molecules. Surfactants are chemicals that self-assemble molecular clusters in a solution (oil or water phase) known as micelles. SLS has been suggested as a potentially effective topical microbicide for intravaginal usage to suppress and potentially prevent infection by various enveloped and non-enveloped viruses. The acoustical parameters such as ultrasonic velocity, density, and viscosity of a solution containing an anionic surfactant (SLS) and alcohols (Anise and Cinnamyl alcohol) are determined at various temperatures (303,313 and 323 K). Similarly, the acoustical parameters such as adiabatic compressibility (b), intermolecular free length (Lf), internal pressure () Rao's constant (Ra), absorption coefficient (a/f2), free volume (Vf), cohesive energy (CE), relaxation time (), acoustic impedance (Za), and solvation number (Sn) were calculated from the observed values. At the end, it is concluded that SLS+water- Anise alcohol has been used as a best additive. This composite has a lower foaming tendency due to lower surface tension, leading to a newer product.
KEYWORDS:Anisyl alcohol; Cinnamyl alcohol; Surfactants; Sodium Lauryl Sulphate; SLS
Download this article as:| Copy the following to cite this article: Aruna S, Thiyagarajan R, Panneerselvam A, Gnanasaravanan S. Acoustical and Spectroscopical Analysis of Surfactant with Different Alcohols at Various Concentrations and Temperatures. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Aruna S, Thiyagarajan R, Panneerselvam A, Gnanasaravanan S. Acoustical and Spectroscopical Analysis of Surfactant with Different Alcohols at Various Concentrations and Temperatures. Orient J Chem 2023;39(4). Available from: https://bit.ly/3YRBGn3 |
Introduction
The study of ultrasonic has become increasingly fascinating in recent years. The ultrasonic study of liquids is significant for comprehending molecular interaction type and intensity. The character of inter- and intra-molecular interactions can be examined in great detail by studying how ultrasonic waves propagate through liquids and liquid mixtures1.
Understanding the chemical interactions in paired mixes depends on the values of the acoustical parameters. A crucial physical parameter with a physical dependence is ultrasonic velocity2,3. Studies on acoustic factors have evolved recently, according to hid4,5. Acoustic characteristics can be used to explain the solute-solvent interaction since they are sensitive to changes. Additionally, the detection and evaluation of both weak and strong molecule interactions have been effectively accomplished using ultrasonic velocity measurements6,7.
Surfactants are chemicals that self-assemble molecular clusters in a solution (oil or water phase) known as micelles and adhere to the contact between a solution and another phase. The surfactants are classified as hydrophilic and hydrophobic depending on how easily they dissolve in water. Both hydrophilic and hydrophobic surfactants dissolve in lipids and water, respectively. The use of surfactants in industrial, agricultural, food, cosmetic, and pharmaceutical applications is widespread. Most of these substances are chemically created and may be poisonous and environmentally harmful8,9.
The anionic surfactant chosen for the current investigation, sodium dodecyl sulphate, has a negative charge on its head group because of their lower degree of soil adsorption than cationic and non-ionic surfactants10.
Material and Methods
Materials
An anionic surfactant and alcohols of AnalaR grade were purchased from SD fine chemicals, Mumbai, India. The experimental solutions were made by dissolving a known weight of anionic surfactant in distilled water and stirring under reflux until clear solutions were achieved. The stock solution was made with double the amount of distilled water. The ultrasonic velocity measurements in SLS with anise alcohol and cinnamyl alcohol were performed in an ultrasonic interferometer (model F81, Mittal Enterprises, New Delhi, India) at a fixed single frequency of 2 MHz and various temperatures (303, 313, and 323 K). The temperature was kept constant using circulating water from a thermostatically controlled (0.1K) water bath. The viscosity was measured with an Ostwald’s viscometer and standardized with double distilled water with an accuracy of 0.001%.
Methods
The velocity of SLS in the presence of different alcohols (anise and cinnamyl alcohols) was determined at various temperatures using an ultrasonic interferometer with a fixed frequency of 2 MHz (303, 313, and 323 K). Densities were determined at a range of temperatures using a specific gravity bottle under standard procedures. The viscosity was obtained with a 0.001 percent accuracy using an Ostwald viscometer calibrated with double distilled water. The various velocity and absorption parameters were calculated to explore the molecular interactions between anionic surfactants and aromatic alcohols11.
Results and discussion
Results
The acoustical parameters such as adiabatic compressibility, free length, internal pressure and Rao’s constant are estimated at all three temperatures from the measured values of ultrasonic velocity, density, and viscosity of the solutions. That are displayed in Tables 1 and 2. Tables 3 and 5.2.4 show the fluctuation of the ultrasonic absorption coefficient with concentrations in the solutions and other computed parameters at 303, 313 and 323 K.
Figures 1 to 6 display plots of SLS concentration vs ultrasonic velocity, viscosity and adiabatic compressibility of alcohols at various temperatures. FTIR analysis of the pure SLS, SLS + anise alcohol and SLS+cinnamyl alcohol spectra was done in a 1:1 ratio. The recorded FTIR spectra are shown in Figures 7 and 8, respectively.
Table 1: Ultrasonic Velocity and Related Acoustical Parameters in the Solution of SLS in Anise Alcohol
|
Temp. K |
Conc |
U |
Ρ kgm-3 |
η |
β N-1m2 |
Lf |
πi Pascal |
R X10-3 |
|
303 |
0.0 |
1412 |
1127 |
1.349 |
4.450 |
0.421 |
1.568 |
2.728 |
|
0.5 |
1419 |
1131 |
1.458 |
4.391 |
0.418 |
2.232 |
2.079 |
|
|
1.0 |
1426 |
1137 |
1.574 |
4.325 |
0.415 |
2.640 |
1.842 |
|
|
1.5 |
1436 |
1144 |
1.682 |
4.239 |
0.411 |
2.976 |
1.717 |
|
|
2.0 |
1447 |
1149 |
1.776 |
4.157 |
0.406 |
3.212 |
1.642 |
|
|
2.5 |
1440 |
1157 |
1.897 |
4.168 |
0.407 |
3.461 |
1.581 |
|
|
313 |
0.0 |
1396 |
1117 |
1.272 |
4.594 |
0.428 |
1.572 |
2.742 |
|
0.5 |
1405 |
1126 |
1.338 |
4.500 |
0.423 |
2.214 |
2.082 |
|
|
1.0 |
1417 |
1131 |
1.402 |
4.434 |
0.419 |
2.596 |
1.848 |
|
|
1.5 |
1428 |
1137 |
1.495 |
4.313 |
0.414 |
2.895 |
1.724 |
|
|
2.0 |
1438 |
1141 |
1.607 |
4.238 |
0.408 |
3.151 |
1.650 |
|
|
2.5 |
1432 |
1146 |
1.715 |
4.255 |
0.412 |
3.387 |
1.593 |
|
|
323 |
0.0 |
1381 |
1107 |
1.218 |
4.648 |
0.434 |
1.587 |
2.756 |
|
0.5 |
1392 |
1113 |
1.282 |
4.590 |
0.430 |
2.229 |
2.099 |
|
|
1.0 |
1406 |
1121 |
1.347 |
4.506 |
0.424 |
2.620 |
1.859 |
|
|
1.5 |
1418 |
1128 |
1.402 |
4.421 |
0.419 |
2.888 |
1.734 |
|
|
2.0 |
1429 |
1133 |
1.483 |
4.364 |
0.410 |
3.119 |
1.659 |
|
|
2.5 |
1422 |
1138 |
1.557 |
4.303 |
0.416 |
3.326 |
1.60 |
Table 2: Ultrasonic velocity and related acoustical parameters in the solution of SLS in Cinnamyl alcohol
|
Temp. K |
Conc |
U |
Ρ kgm-3 |
η |
β N-1m2 |
Lf |
πi Pascal |
R X10-3 |
|
303 |
0.0 |
1360 |
1028 |
1.245 |
5.259 |
0.457 |
1.444 |
2.953 |
|
0.5 |
1372 |
1131 |
1.343 |
4.697 |
0.432 |
2.213 |
2.028 |
|
|
1.0 |
1380 |
1257 |
1.511 |
4.177 |
0.408 |
2.900 |
1.618 |
|
|
1.5 |
1391 |
1291 |
1.621 |
4.003 |
0.399 |
3.296 |
1.475 |
|
|
2.0 |
1385 |
1346 |
1.737 |
3.873 |
0.393 |
3.702 |
1.351 |
|
|
2.5 |
1374 |
1417 |
1.821 |
3.738 |
0.386 |
4.082 |
1.247 |
|
|
313 |
0.0 |
1348 |
1007 |
1.183 |
5.465 |
0.464 |
1.440 |
3.006 |
|
0.5 |
1361 |
1120 |
1.253 |
4.802 |
0.438 |
2.203 |
2.043 |
|
|
1.0 |
1372 |
1177 |
1.321 |
4.514 |
0.424 |
2.686 |
1.725 |
|
|
1.5 |
1384 |
1234 |
1.452 |
4.231 |
0.410 |
3.135 |
1.540 |
|
|
2.0 |
1377 |
1300 |
1.621 |
4.057 |
0.402 |
3.620 |
1.396 |
|
|
2.5 |
1371 |
1381 |
1.834 |
3.852 |
0.392 |
4.164 |
1.273 |
|
|
323 |
0.0 |
1336 |
999 |
1.104 |
5.608 |
0.473 |
1.392 |
3.021 |
|
0.5 |
1347 |
1110 |
1.211 |
4.965 |
0.445 |
2.233 |
2.054 |
|
|
1.0 |
1359 |
1151 |
1.300 |
4.704 |
0.433 |
2.722 |
1.758 |
|
|
1.5 |
1371 |
1201 |
1.421 |
4.430 |
0.419 |
3.158 |
1.578 |
|
|
2.0 |
1365 |
1281 |
1.579 |
4.190 |
0.408 |
3.667 |
1.413 |
|
|
2.5 |
1356 |
1354 |
1.713 |
4.017 |
0.400 |
4.121 |
1.294 |
Table 3: Ultrasonic absorption and related acoustical parameters in the solution of SLS in Anise alcohol
|
Temp |
Conc |
α/f2 X10-15 Np m-1s2 |
Vf X10-15 m3mol-1 |
Cohesive energy X10-8 |
τ X10-12 sec |
za kgm-2 s2 |
Sn |
|
303 |
0.0 |
1.117 |
1.352 |
21.194 |
0.800 |
1.591 |
– |
|
0.5 |
1.186 |
1.021 |
22.794 |
0.853 |
1.605 |
-18.143 |
|
|
1.0 |
1.233 |
0.942 |
24.885 |
0.892 |
1.621 |
-7.242 |
|
|
1.5 |
1.305 |
0.978 |
29.095 |
0.950 |
1.643 |
-2.949 |
|
|
2.0 |
1.341 |
1.006 |
32.318 |
0.984 |
1.662 |
-0.907 |
|
|
2.5 |
1.444 |
1.055 |
36.500 |
1.054 |
1.666 |
-0.808 |
|
|
313 |
0.0 |
1.101 |
1.217 |
19.131 |
0.779 |
1.559 |
– |
|
0.5 |
1.126 |
0.844 |
19.577 |
0.802 |
1.582 |
-25.741 |
|
|
1.0 |
1.146 |
0.805 |
20.906 |
0.823 |
1.603 |
-10.205 |
|
|
1.5 |
1.187 |
0.812 |
23.515 |
0.860 |
1.623 |
-4.857 |
|
|
2.0 |
1.245 |
0.858 |
27.038 |
0.908 |
1.640 |
-2.381 |
|
|
2.5 |
1.340 |
0.899 |
30.451 |
0.972 |
1.641 |
-2.149 |
|
|
323 |
0.0 |
1.098 |
1.122 |
17.800 |
0.770 |
1.529 |
– |
|
0.5 |
1.123 |
0.818 |
18.233 |
0.792 |
1.550 |
-34.958 |
|
|
1.0 |
1.137 |
0.750 |
19.643 |
0.810 |
1.576 |
-14.034 |
|
|
1.5 |
1.146 |
0.729 |
21.080 |
0.824 |
1.600 |
-7.154 |
|
|
2.0 |
1.179 |
0.754 |
23.502 |
0.854 |
1.619 |
-3.908 |
|
|
2.5 |
1.251 |
0.770 |
25.600 |
0.902 |
1.620 |
-3.467 |
Table 4: Ultrasonic absorption and related acoustical parameters in the solution of SLS in Cinnamyl alcohol.
|
Temp |
Conc |
α/f2 X10-15 Np m-1s2 |
Vf X10-15 m3mol-1 |
Cohesive energy X10-8 |
τ X10-12 sec |
za kgm-2 s2 |
Sn |
|
303 |
0 |
1.266 |
1.133 |
16.355 |
0.873 |
1.398 |
– |
|
0.5 |
1.209 |
0.841 |
18.616 |
0.841 |
1.552 |
-37.970 |
|
|
1.0 |
1.203 |
0.843 |
24.413 |
0.841 |
1.735 |
-2.273 |
|
|
1.5 |
1.227 |
0.855 |
28.175 |
0.865 |
1.796 |
2.442 |
|
|
2.0 |
1.277 |
0.882 |
32.636 |
0.897 |
1.864 |
4.103 |
|
|
2.5 |
1.303 |
0.893 |
36.978 |
0.907 |
1.947 |
5.173 |
|
|
313 |
0 |
1.261 |
1.036 |
14.913 |
0.862 |
1.357 |
– |
|
0.5 |
1.167 |
0.749 |
16.497 |
0.805 |
1.524 |
-46.616 |
|
|
1.0 |
1.143 |
0.683 |
18.342 |
0.795 |
1.615 |
-13.941 |
|
|
1.5 |
1.167 |
0.719 |
22.547 |
0.819 |
1.708 |
-2.976 |
|
|
2.0 |
1.256 |
0.788 |
28.937 |
0.877 |
1.790 |
0.766 |
|
|
2.5 |
1.355 |
0.900 |
37.549 |
0.942 |
1.893 |
3.466 |
|
|
323 |
0 |
1.148 |
0.842 |
11.725 |
0.777 |
1.335 |
– |
|
0.5 |
1.174 |
0.701 |
15.645 |
0.802 |
1.495 |
-56.332 |
|
|
1.0 |
1.183 |
0.657 |
17.900 |
0.815 |
1.564 |
-20.541 |
|
|
1.5 |
1.207 |
0.686 |
21.680 |
0.839 |
1.647 |
-7.642 |
|
|
2.0 |
1.274 |
0.748 |
27.415 |
0.882 |
1.749 |
-1.611 |
|
|
2.5 |
1.334 |
0.799 |
23.919 |
0.917 |
1.836 |
1.122 |
 |
Figure 1: Velocity Vs concentration of SLS |
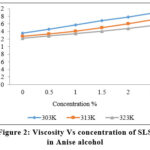 |
Figure 2: Viscosity Vs concentration of SLS |
 |
Figure 3: Adiabatic compressibility Vs concentration of SLS in Anise alcohol |
 |
Figure 4: Ultrasonic velocity Vs concentration |
 |
Figure 5: Viscosity Vs concentration of SLS in Cinnamyl alcohol |
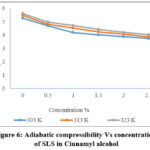 |
Figure 6: Adiabatic compressibility Vs concentration of SLS in Cinnamyl alcohol |
 |
Figure 7: FTIR spectra of Sodium Lauryl Sulphate (SLS) +Anisyl alcohol |
 |
Figure 8: FTIR spectra of Sodium Lauryl Sulphate (SLS) +Cinnamyl alcohol |
Discussion
Ultrasonic velocity against SLS + (Anise alcohol and Cinnamyl alcohol) initially increased and attained a maximum value at CMC and started decreasing with an increase in concentration for the two systems, as given in Figures 1 and 4. This may be due to the micelle formation occurring in the system. The percentages of solvated alcohol molecules per repetition unit could be explained in terms of propagated attractions, as Moore and Uddin (1970) noted12.
It is observed that both density and viscosity increase with an concentration and decrease with increase of temperature. The plot of viscosity versus concentration at three different temperatures is shown in Figures 2 and 4. Aqueous surfactant is homogeneous solution and become more viscous, as the solute is included. The first type of mutual interaction, hydrodynamic screening significantly determines the viscous flow characteristics of diluted surfactant solutions.11.
The decrease in compressibility has been seen in surfactant solutions and is explained by a change in the compressibility of the solvent molecules involved in the solutions12. The decrease of internal pressure with a concentration of SLS + alcohols confirmed the solute-solvent interactions. The closed packing of molecules within the shield imitates the decrease in intermolecular free length and internal pressure13.
From the FTIR studies, the OH stretching bands at around 3445 cm-1 and the bending vibrations at 1656 cm-1 in the pure SLS spectrum demonstrate the vibrational frequencies of the functional groups7. But in SLS mixed AA spectrum, these peaks are shifted to 3392 and 1612 cm‑1 whereas in SLS mixed with CA, the peaks are shifted to 3389 and 1707 cm‑1. The symmetric stretching vibrations of CH3 are detected at 2848 cm-1 and the CH3 asymmetric stretching band is found at 2914 cm-1 in pure SLS. The Peaks are observed at 2849 and 2918 cm-1 in SLS+AA, whereas in SLS+CA, it is observed at 2851 and 2916 cm-1. These peaks indicate the presence of SLS in the complexes. Altogether the presence of SLS with the chemical structure (C12H25NaSO4) in the composites is proved by symmetric stretching at 2843 cm-1 and asymmetric stretching at 2910 cm -1 14. In the investigation, from figure 5.4.2 and 5.4.3, the characteristic peaks observed at 2849 cm-1 and 2918 cm -1 in the SLS+AA system and at 2851 cm-1 and 2916 cm -1 in the SLS+AA system confirms the structure of SLS in alcohol system.
Conclusion
The cleaning agent, surfactant sodium lauryl sulphate (SLS) is primarily utilized in personal care and cleaning products. Anisyl Alcohol is a primary alcohol that belongs to the fragrance structural group Aryl Alkyl Alcohols. Cosmetics and personal care products can benefit from anisyl alcohol as a scent component. The present study reveals that the intermolecular interaction of SLS with alcohols (Anise alcohol and Cinnamyl alcohol) and the existence of SLS and alcohol interaction is taking place through hydrogen bonding with an increase of SLS concentration. Different alcohol groups, the potency of interaction are more in SLS + water – Anise alcohol compared to SLS+water- Cinnamyl alcohol system. Finally, it is concluded that SLS+water- Anise alcohol has been used as the best additive. This composite has a lower foaming tendency due to lower surface tension, leading to a newer product. Additionally, the solvent used in surfactants is less toxic to human society. In this regard, anisyl alcohol is less harmful and toxic to human society when used as a solvent for SLS.
Conflict of Interest
There are no conflict of interest.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Chithralekha, N; Panneerselvam, A. Ultrasonic and UV Analysis on Aqueous Non-Ionic Surfactants. Oriental Journal of Chemistry, 2019. 35(Special Issue 1), 28–35.
CrossRef - Rajathi. K.; Askar Ali. S.J.; Rajendran. A. Ultrasonic study of molecular dynamics in some binary mixtures. J. Chem. Pharm. Res., 2011, 3, 348.
- Palaniappan. L. Ultrasonic Analysis of Intermolecular Interaction in the Mixtures of Benzene with Methanol, Ethanol, 1-propanol. Asian Journal of Material Science, 2012, 4(1), 21-27. DOI: 10.3923/ajmskr.2012.21.27.
CrossRef - Bhandakkar V D; Ind. J. Pure & Appl. Phy., 2011, 49, 250.
- Ishwara Bha. J; Shivakumar. H.R. Study on acoustic behaviour of potassium thiocyanate in aqueous and various non-aqueous solvents at 298 – 313K. Indian J. Chem A., 1998, 37, 252-256.
- Jafta, N.; Mochane, M. J.; Mokhena, T. C.; Lebelo, K. Effect of sodium lauryl sulfate (SLS)/carbon nanotubes on the properties of cellulose membrane isolated from maize stalk. Cell. Chem. and tech. 2022, 56(5-6), 549-558. DOI: https://doi.org/10.35812/CelluloseChemTechnol. 2022.56.47
- Karikalan, V.; Panneerselvam. A..; Vallalperuman, K., Physico – Chemical Analysis on Cetylpyridinium Chloride (CPC) With Alcohol Solution at Different Temperatures – Ultrasonic, UV And FTIR Analysis. Digest Journal of Nanomaterials and Biostructures, 2018,13(1), 115-128.
- Makkar, R. S.; Rockne, K. J. Comparison of synthetic surfactants and biosurfactants in enhancing biodegradation of polycyclic aromatic hydrocarbons. Environmental Toxicology and Chemistry, 2003, 22(10), 2280-2292. DOI: 10.1897/02-472.
CrossRef - Schramm, L. L.; Stasiuk, E. N.; Marangoni, D. G. Surfactants and their applications. Annu. Rep. Prog. Chem., Sect. C: Phys. Chem., 2003, 99, 3–48. https://doi.org/10.1039/B208499F.
CrossRef - Punitha. S,; Uvarani. R. Molecular interactions of surfactants with polymer in aqueous solutions. Journal of Chemical and Pharmaceutical Research,2012, 4 (1), 387-392.
- Bell, W.; North, A.M.; Pethrick, R.A.; Teik. Ultrasonic relaxation studies of chain entanglement effects in poly(dimethylsiloxanes). J. Chem. Soc. Faraday Trans.2, 1979,1115. DOI:10.1039/f29797501115
CrossRef - Moore, W.R.; Uddin, M.A. Dilute solution properties of bisphenol a polycarbonate—III.: Adiabatic compressibilities of polymer solutions. Euro. Polym. J., 1970, 6, 547-551.
CrossRef - A. Gupta and S. Upadhyaya, “Acoustical behaviour of cerium and thorium myristate in mixed organic solvents “, Cellulose Chemistry Technology, 47, (2013), 77-82.
- Viana, R. B.; da Silva, A.B. F., & Pimentel, A. S. Infrared Spectroscopy of Anionic, Cationic, and Zwitterionic Surfactants. Advances in Physical Chemistry, 2012, 2012, 1–14.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









