Ultrasonic Studies in the Binary Mixtures of O - Chlorophenol with Salicylates at Different Temperatures
D. V. M. Krishna Reddy1, P. B. Sandhya Sri2 , K. A. K. Raj Kumar3 and L. Vaikunta Rao1*
1Department of Chemistry, Gitam University-530045, Visakhapatnam.
2Department of Physics, Government Degree College, Avanigadda – 521121, Krishna Andhra Pradesh, India.
3Dr. L. B. College, Vishakhapatnam-530013, Andhra Pradesh, India.
Corresponding Author E-mail: sandhyasri.prathipati@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390213
Article Received on : 13 Dec 2022
Article Accepted on : 31 Mar 2023
Article Published : 28 Apr 2023
Reviewed by: Dr. Babu Shaik,
Second Review by: Dr. B. B. Nanda
Final Approval by: Dr. Umesh Chandra Sharma
The ultrasonic velocities (U), densities (ρ) and viscosities (η) of Binary Mixture O - Chlorophenol + methyl salicylate, + ethyl salicylate and + benzyl salicylate from 303.15 to 318.15K. Excess molar volume (VE), deviation in adiabatic compressibility (Δβad) and excess inter molecular free length (LfE) have been calculated from the measured experimental data. The values of VE, Δβad, LfE and Δη have been fitted to Redlich – Kister polynomial equation to estimate binary coefficients and standard deviation between the experimental and computed values. Using Nomoto's relation (UNR), impedance relation (UIR), ideal mixing relation (Uimx), Junjie's relation (UJ), Rao’s specific velocity relation (UR) and Kudriavtsev relation (UK), the theoretical ultrasonic velocities were evaluated. The computed estimations of ratio in velocity (U2/U2imx) from measured estimations of (U) are graphically shown. The molecular interaction parameter (α) has been evaluated. The validity of the theories were checked by calculating standard deviation and chi square test.
KEYWORDS:Density; Excess Parameters; Theoretical Velocities; Ultrasonic velocity; Viscosity
Download this article as:| Copy the following to cite this article: Reddy D. V. M. K, Sri P. B. S, Kumar K. A. K. R, Rao L. V. Ultrasonic Studies in the Binary Mixtures of O - Chlorophenol with Salicylates at Different Temperatures. Orient J Chem 2023;39(2). |
| Copy the following to cite this URL: Reddy D. V. M. K, Sri P. B. S, Kumar K. A. K. R, Rao L. V. Ultrasonic Studies in the Binary Mixtures of O - Chlorophenol with Salicylates at Different Temperatures. Orient J Chem 2023;39(2). Available from: https://bit.ly/3niySks |
Introduction
Molecular studies lie in the ability to assess the information stored in the structure of the molecule as a function of their physical and chemical properties. There had been many developments in Chemistry and Physics during the past two centuries particularly, in the fields of statistical mechanics, thermodynamics and the nature of the chemical bond. The ensuing atomistic view shall be presented and discussed in the context of molecular interactions. Molecular interactions are generally electrostatic in nature. The strength of these interactions and the forces among atoms can be analyzed according to their thermodynamic and kinetic behaviour1,2.
Ultrasonic velocities have been adequately employed in understanding the nature of molecular interaction in pure liquids and binary mixtures3,4. The method of studying the molecular interaction from the knowledge of the variation of thermodynamic parameters and their excess values with composition gives an insight into the molecular process5,6. The present paper is part of our research work on the thermodynamic properties of liquid-liquid mixtures7-9. This paper includes the density and ultrasonic behaviour of binary mixtures of o – chlorophenol with methyl salicylate, ethyl salicylate and benzyl salicylate over the entire composition range at 308.15 K.
As the studied compounds have a plenty of industrial and other applications, the author made an attempt to study the thermodynamic behaviour of the binary mixtures of the compound
A literature survey10,11 reveals that molecular interactions in O – chlorophenol with various compounds were studied over the entire range of composition at different temperatures.
Study of molecular interactions for the binary mixtures of salicylates was done by many researchers12,13.
Keeping these important aspects in mind, the present study deals with the ultrasonic and thermodynamic study of O – chlorophenol (OCP) with higher salicylates (methyl salicylate. Ethyl salicylate and benzyl salicylate) over the entire mole fraction range from 303.15 to 318.15K. The liquids under investigation have been chosen based on their multifold applications. Ultrasonic velocities, densities and viscosities of the studied binary mixtures are measured and using the experimental data, excess molar volume, deviation in adiabatic compressibility, excess inter-molecular free length and deviation in viscosity are reported in this paper. The results have been used to estimate the molecular interactions in the constituent molecules.
Experimental Techniques
Ultrasonic velocities(U) and densities (ρ)of pure liquids and all liquid mixtures in the present study were measured by employing a pulse-echo interferometer (MODEL M-81) supplied by the Mittal Enterprises, New Delhi and a 10−5 m3 double-arm pycnometer respectively at (303.15, 308.15, 313.15 and 318.15)K as described by Nikam et al.[14]. The Double-arm pycnometer is calibrated using conductivity water with 995.61 kg m−3 as its density at 303.15 K. To maintain the constant temperature, a thermostat is employed with an accuracy of±0.01K. The weighing is carried out using METTLER TOLEDO (Switzerland make) ABB5-S/F ACT digital balance with an accuracy of ±0.01mg. The viscosity was measured using a commercial Ubbelohde capillary viscometer of 0.55 mm diameter calibrated with double distilled water at temperatures of 303.15, 308.15, 313.15 and 318.15K. The experimental samples, O – chlorophenol, salicylates such as methyl salicylate, ethyl salicylate and benzyl salicylate used in the present study are of AR grade quality, obtained from S.D. Fine chemicals, India with purity > 99%. Experimental values are compared with literature values and are shown in Table 111&15. It is evident fromTable1, that there is good agreement between the experimental and reported values.
Table 1: Comparison of Experimental Values with Literature Data11,15 at 303.15K
|
Liquid |
Density Kg/m3 |
Ultrasonic Velocity m/s |
Viscosity η cP |
|||
|
Exptl |
Lit |
Exptl |
Lit |
Exptl |
Lit |
|
|
1-OCP |
1.1423 |
1.2550a |
1365.2 |
1381.4a |
2.823 |
2.821a |
|
Methyl Salicylate |
1.1752 |
1.1750b |
1389.5 |
1390.4b |
2.443 |
2.44b |
|
Ethyl Salicylate |
1.1181 |
1.118b |
1355.5 |
1354.4b |
3.175 |
3.117b |
|
Benzyl Salicylate |
1.1063 |
1.1065b |
1498.3 |
1498.4b |
6.733 |
6.722b |
Mean molar volume (V) evaluated by the equation
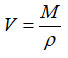
Excess volume (VE): VE = V — (V1X1+V2X2)
Adiabatic compressibility (βad): βad = 1/P U2
Deviation in adiabatic compressibility (Δβad): (Δβad) = βad — ( βad1 X1 + βad2 X2 )
Inter molecular free length (Lf ): Lf = K (βad )1/2
Excess inter molecular free length (LfE): LfE = Lf ( Lf1 X1 + Lf2 X2
Where, Lf1 and Lf2 are the individual inter molecular free length values of pure liquids in the binary mixtures.
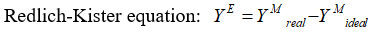
The standard deviations σ (YE) were calculated by using the relation
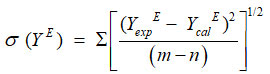
Where, m is the number of experimental data points and n is the number of coefficients considered and (n=5 in the present calculation).
Ycal E has been obtained from the above equation using the best – fit values of Ai.
Theoretical Velocities
Nomoto Equation
following relation established by Nomoto17 for a liquid mixture
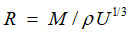
Where U and P are experimentally determined values of Ultrasonic velocity and density respectively and M indicates the mean molar mass in a binary liquid mixture
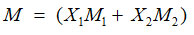
where M1 and M2 are molecular weights of molecules of constituent components.
On simplification, we get the following relation.
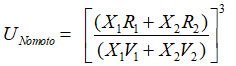
The Van Dael and Vangeel Equation
The ideal mixing theory advanced by Van Dael and Vangeel18 through light of assumptions of Blandamer and Waddington19 Van Dael obtained the relation for ultrasonic velocity in liquid mixtures as
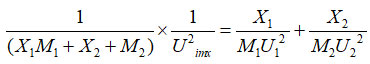
where Uimx is the ideal mixing ultrasonic velocity in binary liquid mixture.U1 and U2 are ultrasonic velocities of main compound and sub compound.
The Impedance relation
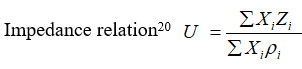
where Xi, ri and Zi are mole fraction, density and acoustic impedance of the mixture respectively.
The Rao’s specific velocity method relation
Rao developed the following equation namely specific velocity method21
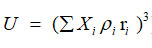
where Xi ,Ui, pi and ri are the mole fraction, ultrasonic velocity, density and Rao’s specific sound velocity of the mixture.
Where Rao’s specific sound velocity = Ui1/3/pi and Zi is the acoustic impedance.
The Junjie equation
Junjie equation22
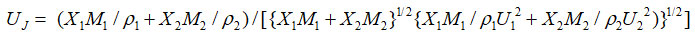
where M1, M2 and p1, p2 are molecular weights and densities of constituent components.
Kudriavtsev Equation
Kudriavtsev Relation23 for the ultrasonic relation between experimental and theoretical values are calculated
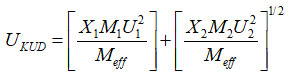
Chi Square Test
As indicated by Karl Pearson24, Chi-square value is evaluated for the binary liquid mixtures under study utilizing the formula
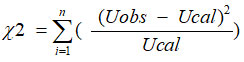
where n is the number of data used, and U (obs) = experimental values of ultrasonic velocities U(cal) = computed values of ultrasonic velocities.
Average Percentage Error
The Average percentage error[25] is calculated by utilizing the relation
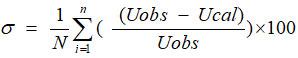
where n is the number of data used. U (obs) = experimental values of ultrasonic velocities
The degree of intermolecular interaction or molecular association is given by
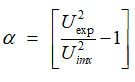
Results
The experimentally measured values of ultrasonic velocities (U), densities (ρ) and viscosities are presented in the table 2. From the table it is clear that experimental values are in good agreement with the literature values.
Table 2 : Ultrasonic velocity, density, viscosity and excess molar volume, Excess intermolecular free length, deviations in viscosity and adiabatic compressibility of binary mixtures of OCP with mehyl salicylate, ethyl salicylate and benzyle salycilate at 303.15, 308.15,313.15 and 318.15K
|
OCP + Methyl Salicylate 303.15K |
||||||||||||||||||||||||||||||
|
0.0000 0.1231 0.2401 0.3514 0.4573 0.5583 0.6547 0.7468 0.8349 0.9192 1.0000 |
1389.50 1395.80 1405.20 1411.90 1417.80 1423.00 1417.50 1410.00 1400.00 1390.00 1382.00 |
1.1752 1.1848 1.1939 1.2027 1.2111 1.2192 1.2270 1.2344 1.2416 1.2485 1.2551 |
2.4404 2.8750 3.2177 3.4816 3.6333 3.6814 3.6200 3.5052 3.3138 3.0821 2.8230 |
44.0728 43.3227 42.4169 41.7087 41.0750 40.5054 40.5625 40.7478 41.0935 41.4568 41.7163 |
129.4673 125.9686 122.6905 119.6131 116.7188 113.9920 111.4186 108.9864 106.6840 104.5016 102.4301 |
1.3175 1.3063 1.2925 1.2817 1.2719 1.2631 1.2640 1.2669 1.2722 1.2778 1.2818 |
0.0000 -0.4599 -1.0901 -1.5361 -1.9202 -2.2518 -1.9676 -1.5652 -1.0119 -0.4499 0.0000 |
0.0000 -0.1692 -0.2848 -0.3543 -0.3844 -0.3806 -0.3477 -0.2899 -0.2107 -0.1132 0.0000 |
0.0000 -0.0069 -0.0164 -0.0233 -0.0293 -0.0345 -0.0302 -0.0240 -0.0155 -0.0069 0.0000 |
0.000 0.387 0.685 0.907 1.018 1.027 0.929 0.779 0.554 0.290 0.000 |
||||||||||||||||||||
|
308.15K |
||||||||||||||||||||||||||||||
|
0.0000 0.1231 0.2401 0.3514 0.4573 0.5583 0.6547 0.7468 0.8349 0.9192 1.0000 |
1374.40 1383.00 1392.00 1397.20 1403.10 1407.10 1401.00 1393.00 1383.00 1373.50 1361.20 |
1.1685 1.1782 1.1873 1.1960 1.2044 1.2124 1.2201 1.2276 1.2348 1.2417 1.2481 |
2.2284 2.5700 2.9100 3.1497 3.2831 3.3130 3.2608 3.1210 2.9381 2.6999 2.4950 |
45.3049 44.3767 43.4664 42.8296 42.1759 41.6582 41.7559 41.9795 42.3415 42.6896 43.2421 |
130.2097 126.6774 123.3751 120.2834 117.3749 114.6310 112.0421 109.5896 107.2705 105.0698 103.0046 |
1.3358 1.3221 1.3084 1.2988 1.2889 1.2809 1.2824 1.2859 1.2914 1.2967 1.3051 |
0.0000 -0.6742 -1.3432 -1.7505 -2.1857 -2.4950 -2.1985 -1.7849 -1.2412 -0.7191 0.0000 |
0.0000 -0.1821 -0.3022 -0.3674 -0.3939 -0.3902 -0.3567 -0.3037 -0.2265 -0.1331 0.0000 |
0.0000 -0.0100 -0.0200 -0.0262 -0.0329 -0.0377 -0.0332 -0.0270 -0.0187 -0.0108 0.0000 |
0.000 0.309 0.618 0.828 0.933 0.936 0.858 0.694 0.487 0.226 0.000 |
||||||||||||||||||||
|
313.15K |
||||||||||||||||||||||||||||||
|
0.0000 0.1231 0.2401 0.3514 0.4573 0.5583 0.6547 0.7468 0.8349 0.9192 1.0000 |
1358.00 1369.50 1379.00 1384.00 1390.00 1394.00 1388.80 1379.70 1369.50 1359.50 1345.20 |
1.1678 1.1770 1.1856 1.1937 1.2014 1.2088 1.2160 1.2229 1.2295 1.2357 1.2413 |
2.2284 2.4800 2.7548 2.9858 3.0737 3.0810 2.9982 2.8416 2.6591 2.4200 2.2570 |
46.4336 45.3021 44.3540 43.7354 43.0814 42.5702 42.6374 42.9583 43.3665 43.7857 44.5194 |
130.2877 126.8066 123.5540 120.5172 117.6666 114.9696 112.4235 110.0135 107.7329 105.5816 103.5688 |
1.3524 1.3358 1.3217 1.3125 1.3026 1.2949 1.2959 1.3008 1.3069 1.3132 1.3242 |
0.0000 -0.8958 -1.6199 -2.0257 -2.4768 -2.7947 -2.5430 -2.0458 -1.4690 -0.8884 0.0000 |
0.0000 -0.1908 -0.3181 -0.3825 -0.4026 -0.4011 -0.3716 -0.3209 -0.2481 -0.1462 0.0000 |
0.0000 -0.0100 -0.0200 -0.0262 -0.0329 -0.0377 -0.0332 -0.0270 -0.0187 -0.0108 0.0000 |
0.000 0.248 0.520 0.747 0.832 0.837 0.751 0.592 0.407 0.165 0.000 |
||||||||||||||||||||
|
318.15K |
||||||||||||||||||||||||||||||
|
0.0000 0.1231 0.2401 0.3514 0.4573 0.5583 0.6547 0.7468 0.8349 0.9192 1.0000 |
1343.40 1355.80 1365.50 1371.30 1377.00 1381.10 1374.50 1365.00 1354.00 1341.00 1324.10 |
1.1645 1.1733 1.1814 1.1890 1.1962 1.2032 1.2099 1.2162 1.2223 1.2281 1.2330 |
2.0650 2.2579 2.4806 2.7147 2.7938 2.8003 2.6972 2.5497 2.3496 2.1683 2.0390 |
47.5828 46.3672 45.3951 44.7253 44.0888 43.5732 43.7501 44.1285 44.6257 45.2802 46.2589 |
130.6569 127.2043 123.9901 120.9936 118.1762 115.5104 112.9941 110.6150 108.3657 106.2340 104.2660 |
1.3690 1.3514 1.3372 1.3273 1.3178 1.3100 1.3127 1.3184 1.3258 1.3355 1.3498 |
0.0000 -1.0526 -1.8698 -2.3923 -2.8885 -3.2705 -2.9660 -2.4656 -1.8518 -1.0857 0.0000 |
0.0000 -0.2027 -0.3299 -0.3905 -0.4121 -0.4126 -0.3850 -0.335, -0.2582 -0.1645 0.0000 |
0.0000 -0.0131 -0.0239 -0.0300 -0.0368 -0.0418 -0.0380 -0.0306 -0.0219 -0.0132 0.0000 |
0.000 0.196 0.422 0.659 0.741 0.750 0.649 0.504 0.306 0.127 0.000 |
||||||||||||||||||||
|
OCP + Ethyl Salicylate |
||||||||||||||||||||||||||||||
|
303.15K |
||||||||||||||||||||||||||||||
|
0.0000 0.1280 0.2482 0.3614 0.4682 0.5691 0.6645 0.7550 0.8408 0.9224 1.0000 |
1355.50 1365.90 1378.30 1389.50 1399.00 1406.90 1405.00 1402.50 1395.60 1387.70 1382.00 |
1.1181 1.1317 1.1456 1.1594 1.1730 1.1868 1.2003 1.2138 1.2275 1.2410 1.2551 |
3.1770 3.4951 3.7207 3.8873 3.9448 3.8917 3.7910 3.5871 3.3759 3.1020 2.8230 |
48.677 47.362 45.951 44.675 43.558 42.569 42.204 41.884 41.827 41.845 41.716 |
148.618 142.580 136.908 131.606 126.651 121.981 117.618 113.507 109.610 105.947 102.430 |
1.385 1.366 1.345 1.327 1.310 1.295 1.289 1.284 1.284 1.284 1.282 |
0.0000 -0.4239 -0.9977 -1.4857 -1.8600 -2.1465 -1.8470 -1.5378 -0.9973 -0.4118 0.0000 |
0.0000 -0.1284 -0.2462 -0.3195 -0.3427 -0.3528 -0.3069 -0.2398 -0.1720 -0.0680 0.0000 |
0.0000 -0.0057 -0.0138 -0.0210 -0.0267 -0.0313 -0.0270 -0.0226 -0.0147 -0.0060 0.0000 |
0.000 0.363 0.632 0.838 0.934 0.916 0.849 0.677 0.497 0.252 0.000 |
||||||||||||||||||||
|
308.15K |
||||||||||||||||||||||||||||||
|
0.0000 0.1280 0.2482 0.3614 0.4682 0.5691 0.6645 0.7550 0.8408 0.9224 1.0000 |
1338.60 1350.00 1362.00 1373.00 1382.00 1389.00 1387.00 1383.50 1378.00 1368.40 1361.20 |
1.1153 1.1286 1.1422 1.1556 1.1689 1.1823 1.1954 1.2085 1.2217 1.2347 1.2481 |
2.2530 2.5270 2.7480 2.9190 3.0287 3.0753 3.0610 2.9570 2.8080 2.6571 2.4950 |
50.039 48.617 47.196 45.904 44.793 43.840 43.484 43.231 43.107 43.253 43.242 |
148.991 142.969 137.310 132.033 127.095 122.446 118.101 114.005 110.134 106.487 103.005 |
1.404 1.384 1.363 1.345 1.328 1.314 1.309 1.305 1.303 1.305 1.305 |
0.0000 -0.5525 -1.1560 -1.6783 -2.0640 -2.3313 -2.0378 -1.6765 -1.2167 -0.5170 0.0000 |
0.0000 -0.1382 -0.2677 -0.3383 -0.3658 -0.3763 -0.3317 -0.2672 -0.1904 -0.0871 0.0000 |
0.0000 -0.0074 -0.0159 -0.0235 -0.0294 -0.0336 -0.0295 -0.0244 -0.0178 -0.0075 0.0000 |
0.000 0.243 0.435 0.579 0.662 0.685 0.647 0.521 0.352 0.181 0.000 |
||||||||||||||||||||
|
313.15K |
||||||||||||||||||||||||||||||
|
0.0000 0.1280 0.2482 0.3614 0.4682 0.5691 0.6645 0.7550 0.8408 0.9224 1.0000 |
1324.00 1336.00 1348.50 1359.50 1368.00 1374.60 1373.00 1369.00 1364.00 1353.70 1345.20 |
1.1080 1.1215 1.1352 1.1486 1.1620 1.1754 1.1886 1.2017 1.2150 1.2281 1.2413 |
2.2530 2.4699 2.6507 2.7695 2.8562 2.8790 2.8279 2.7028 2.5586 2.3894 2.2570 |
51.485 49.956 48.445 47.106 45.986 45.026 44.630 44.401 44.238 44.435 44.519 |
149.973 143.875 138.163 132.838 127.850 123.164 118.776 114.650 110.738 107.059 103.569 |
1.424 1.403 1.381 1.362 1.346 1.332 1.326 1.322 1.320 1.323 1.324 |
0.0000 -0.6385 -1.3120 -1.8622 -2.2385 -2.4955 -2.2268 -1.8248 -1.3903 -0.6254 0.0000 |
0.0000 -0.1600 -0.2930 -0.3644 -0.3974 -0.4016 -0.3603 -0.2886 -0.2174 -0.1115 0.0000 |
0.0000 0.0074, -0.0159 -0.0235 -0.0294 -0.0336 -0.0295 -0.0244 -0.0178 -0.0075 0.0000 |
0.000 0.216 0.397 0.515 0.601 0.624 0.572 0.447 0.302 0.133 0.000 |
||||||||||||||||||||
|
318.15K |
||||||||||||||||||||||||||||||
|
0.0000 0.1280 0.2482 0.3614 0.4682 0.5691 0.6645 0.7550 0.8408 0.9224 1.0000 |
1306.80 1324.00 1341.50 1357.00 1370.00 1380.50 1383.90 1384.00 1382.00 1376.00 1369.00 |
1.1050 1.1183 1.1315 1.1444 1.1572 1.1702 1.1829 1.1955 1.2082 1.2207 1.2330 |
2.0060 2.1800 2.3459 2.4585 2.5366 2.5607 2.5160 2.4105 2.2793 2.1279 2.0390 |
52.993 51.012 49.112 47.455 46.041 44.840 44.141 43.670 43.336 43.267 43.274 |
150.380 144.290 138.615 133.331 128.379 123.712 119.349 115.245 111.361 107.708 104.266 |
1.445 1.417 1.391 1.367 1.347 1.329 1.319 1.311 1.306 1.305 1.306 |
0.0000 -0.7379 -1.4695 -2.0257 -2.4016 -2.6223 -2.3937 -1.9861 -1.4857 -0.7618 0.0000 |
0.0000 -0.1900 -0.3203 -0.3830 -0.4109 -0.4265 -0.3878 -0.3201 -0.2452 -0.1372 0.0000 |
0.0000 -0.0085 -0.0179 -0.0258 -0.0315 -0.0355 -0.0319 -0.0262 -0.0201 -0.0090 0.0000 |
0.000 0.170 0.332 0.441 0.515 0.536 0.488 0.380 0.246 0.091 0.000 |
||||||||||||||||||||
|
OCP + Benzyl Salicylate 303.15K |
||||||||||||||||||||||||||||||
|
0.0000 0.1692 0.3143 0.4400 0.5500 0.6470 0.7333 0.8105 0.8800 0.9429 1.0000 |
1498.40 1489.00 1484.00 1479.00 1474.00 1468.00 1451.00 1435.00 1415.00 1396.00 1382.00 |
1.1063 1.1206 1.1354 1.1502 1.1651 1.1801 1.1949 1.2097 1.2246 1.2395 1.2551 |
6.7322 6.3449 5.9339 5.6019 5.2100 4.7990 4.3900 3.9700 3.5600 3.1500 2.8230 |
40.260 40.249 39.993 39.746 39.504 39.321 39.749 40.144 40.784 41.398 41.716 |
206.309 188.625 173.432 160.306 148.845 138.754 129.836 121.888 114.750 108.315 102.430 |
1.259 1.259 1.255 1.251 1.247 1.244 1.251 1.257 1.267 1.277 1.282 |
0.0000 -0.2568 -0.7246 -1.1549 -1.5568 -1.8808 -1.5788 -1.2965 -0.7572 -0.2347 0.0000 |
0.0000 -0.1069 -0.2323 -0.2997 -0.3341 -0.3419 -0.2976 -0.2258 -0.1469 -0.0515 0.0000 |
0.0000 -0.0040 -0.0113 -0.0180 -0.0243 -0.0294 -0.0246 -0.0201 -0.0117 -0.0036 0.0000 |
0.000 0.274 0.430 0.590 0.628 0.596 0.524 0.406 0.268 0.104 0.000 |
||||||||||||||||||||
|
308.15K |
||||||||||||||||||||||||||||||
|
0.0000 0.1692 0.3143 0.4400 0.5500 0.6470 0.7333 0.8105 0.8800 0.9429 1.0000 |
1485.90 1477.00 1470.00 1465.10 1458.30 1450.00 1434.40 1415.60 1398.10 1377.00 1361.20 |
1.1030 1.1171 1.1316 1.1461 1.1606 1.1752 1.1897 1.2041 1.2186 1.2331 1.2481 |
6.0800 5.7069 5.2898 4.8980 4.5561 4.2092 3.8480 3.4592 3.1001 2.7500 2.4950 |
41.062 41.034 40.897 40.648 40.515 40.470 40.853 41.444 41.982 42.769 43.242 |
206.927 189.215 174.022 160.879 149.419 139.328 130.406 122.456 115.315 108.875 103.005 |
1.272 1.271 1.269 1.265 1.263 1.263 1.268 1.278 1.286 1.298 1.305 |
0.0000 -0.3970 -0.8504 -1.3731 -1.7462 -2.0024 -1.8080 -1.3852 -0.9986 -0.3490 0.0000 |
0.0000 -0.1259 -0.2460 -0.3246 -0.3533 -0.3577 -0.3138 -0.2405 -0.1615 -0.0687 0.0000 |
0.0000 -0.0061 -0.0130 -0.0211 -0.0268 -0.0308 -0.0277 -0.0211 -0.0152 -0.0053 0.0000 |
0.000 0.234 0.336 0.395 0.448 0.449 0.397 0.285 0.175 0.050 0.000 |
||||||||||||||||||||
|
313.15K |
||||||||||||||||||||||||||||||
|
0.0000 0.1692 0.3143 0.4400 0.5500 0.6470 0.7333 0.8105 0.8800 0.9429 1.0000 |
1471.80 1463.00 1456.70 1452.20 1444.10 1435.20 1419.50 1402.00 1383.60 1361.70 1345.20 |
1.0995 1.1134 1.1276 1.1419 1.1561 1.1704 1.1845 1.1986 1.2128 1.2269 1.2413 |
5.3748 5.0681 4.7269 4.3950 4.0800 3.7700 3.4350 3.1002 2.7650 2.4957 2.2570 |
41.986 41.962 41.793 41.528 41.477 41.480 41.899 42.446 43.072 43.957 44.519 |
207.585 189.844 174.631 161.478 150.001 139.904 130.980 123.018 115.867 109.427 103.569 |
1.286 1.286 1.283 1.279 1.278 1.278 1.285 1.293 1.302 1.316 1.324 |
0.0000 -0.4525 -0.9892 -1.5731 -1.9029 -2.1451 -1.9451 -1.5937 -1.1436 -0.4177 0.0000 |
0.0000 -0.1398 -0.2655 -0.3430 -0.3785 -0.3791 -0.3286 -0.2607 -0.1847 -0.0858 0.0000 |
0.0000 -0.0061 -0.0130 -0.0211 -0.0268 -0.0308 -0.0277 -0.0211 -0.0152 -0.0053 0.0000 |
0.000 0.221 0.332 0.392 0.420 0.413 0.347 0.252 0.134 0.061 0.000 |
||||||||||||||||||||
|
318.15K |
||||||||||||||||||||||||||||||
|
0.0000 0.1692 0.3143 0.4400 0.5500 0.6470 0.7333 0.8105 0.8800 0.9429 1.0000 |
1457.00 1447.00 1441.10 1432.50 1425.50 1415.20 1398.00 1381.10 1363.00 1342.50 1324.10 |
1.0950 1.1088 1.1227 1.1365 1.1504 1.1644 1.1782 1.1919 1.2057 1.2194 1.2330 |
5.4540 5.0082 4.5800 4.1792 3.8280 3.4908 3.1520 2.8470 2.5350 2.2581 2.0390 |
43.020 43.073 42.889 42.879 42.779 42.881 43.428 43.986 44.645 45.501 46.259 |
208.438 190.632 175.394 162.238 150.753 140.625 131.678 123.709 116.549 110.101 104.266 |
1.302 1.303 1.300 1.300 1.298 1.300 1.308 1.316 1.326 1.339 1.350 |
0.0000 -0.4943 -1.1484 -1.5662 -2.0217 -2.2348 -1.9674 -1.6596 -1.2255 -0.5723 0.0000 |
0.0000 -0.1789 -0.3074 -0.3673 -0.3934 -0.4104 -0.3692 -0.2970 -0.2192 -0.1188 0.0000 |
0.0000 -0.0068 -0.0150 -0.0239 -0.0289 -0.0325 -0.0294 -0.0240 -0.0171 -0.0062 0.0000 |
0.000 0.132 0.199 0.228 0.252 0.246 0.202 0.161 0.086 0.024 0.000 |
||||||||||||||||||||
In order to support the presence of interaction between the molecules, it is necessary to study the excess parameters. Excess parameters, associated with a liquid mixture, are a quantitative measure of deviation in the behaviour of the liquid mixture from ideality. The nature and excess functions’ sign can be explained in terms of the molecular interactions considering both the positive and negative contributions. The excess values of molar volume (VE) and inter molecular free length (LfE) and deviation in adiabatic compressibility (Δβad) and viscosity(Δη) are also showed in table 2. Redlich-Kister coefficients along with the standard deviations for all the excess and deviation properties at all the experimental temperatures are presented in Table 3. The non-rectilinear behaviour of ultrasonic velocity, compressibility and other thermo-dynamical parameters of liquid mixtures with changing mole fractions are attributed to the difference in the size of the molecules and strength of interactions.
Table 3: Values of Redlich – Kister coefficients and standard deviation (σ) for for the binary systems OCP + Methyl Salicylate, OCP + Ethyl Salicylate and OCP + Benzyl Salicylate
|
|
A0 |
A1 |
A2 |
A3 |
A4 |
σ |
|
OCP + Methyl Salicylate |
||||||
|
Deviation in Adiabatic Compressibility |
||||||
|
303.15K 308.15K 313.15K 318.15K |
-8.7652 -9.7692 -10.9699 -12.8104 |
4.3983 4.3597 4.4680 5.2435 |
10.3075 11.4484 11.3003 11.4075 |
-5.0538 -4.2453 -4.5802 -4.7023 |
-7.7485 -13.7768 -15.9644 -16.5977 |
0.1014 0.1163 0.1224 0.1244 |
|
Excess Molar Volume |
||||||
|
303.15K 308.15K 313.15K 318.15K |
-1.5466 -1.5887 -1.6328 -1.6781 |
-0.0272 -0.0657 -0.0914 -0.0723 |
0.0007 -0.0686 -0.2860 -0.2083 |
0.0003 0.1575 0.2892 0.3299 |
-0.0013 -0.2374 -0.0825 -0.5190 |
0.00003 0.0023 0.0026 0.0053 |
|
Excess Int. Mole Free Length |
||||||
|
303.15K 308.15K 313.15K 318.15K |
-0.1341 -0.1474 -0.1637 -0.1888 |
0.0699 0.0682 0.0692 0.0797 |
0.1612 0.1766 0.1729 0.1732 |
-0.0800 -0.0662 -0.0703 -0.0718 |
-0.1222 -0.2116 -0.2418 -0.2482 |
0.0016 0.0018 0.0019 0.0019 |
|
Deviation in Viscosity |
||||||
|
303.15K 308.15K 313.15K 318.15K |
4.1358 3.7776 3.4005 3.0576 |
-0.3309 -0.2402 -0.0632 -0.0475 |
-0.7031 -0.7305 -1.0153 -1.6123 |
0.1311 -0.0539 -0.1731 -0.1533 |
0.1268 -0.9200 -1.2557 -0.6975 |
0.0065 0.0055 0.0138 0.0175 |
|
OCP + Ethyl Salicylate |
||||||
|
303.15K 308.15K 313.15K 318.15K |
-8.2366 -9.0287 -9.7323 -10.2698 |
4.8777 4.6353 4.3177 3.9598 |
7.5927 7.9376 7.5847 6.2431 |
-5.3047 -4.9068 -3.7933 -2.3928 |
-3.9493 -5.6939 -6.0017 -5.7436 |
0.0824 0.0865 0.0901 0.0618 |
|
Excess Molar Volume |
||||||
|
303.15K 308.15K 313.15K 318.15K |
-1.4414 -1.5294 -1.6390 -1.7271 |
0.0624 0.1054 0.0685 0.1493 |
0.5701 0.4799 0.5761 0.4490 |
-0.1989 -0.1149 -0.0018 -0.1348 |
0.0343 -0.0197 -0.5358 -0.9130 |
0.0099 0.0085 0.0086 0.0108 |
|
Excess Int. Mole Free Length |
||||||
|
303.15K 308.15K 313.15K 318.15K |
-0.1191 -0.1293 -0.1376 -0.1419 |
0.0780 0.0735 0.0682 0.0652 |
0.1137 0.1178 0.1114 0.0911 |
-0.0835 -0.0759 -0.0584 -0.0380 |
-0.0597 -0.0852 -0.0890 -0.0852 |
0.0013 0.0013 0.0013 0.0009 |
|
Deviation in Viscosity |
||||||
|
303.15K 308.15K 313.15K 318.15K |
3.7254 2.7184 2.4773 2.1222 |
-0.1582 -0.6357 -0.6623 -0.5968 |
-0.6422 -0.6020 -1.1356 -0.8493 |
-0.0912 0.5741 1.0349 0.9893 |
0.1265 0.0954 0.5015 -0.2997 |
0.0136 0.0072 0.0096 0.0068 |
|
OCP + Benzyl Salicylate Deviation in Ad Comp |
||||||
|
303.15K 308.15K 313.15K 318.15K |
-5.8022 -6.6350 -7.2381 -7.5019 |
9.3881 8.7182 7.3829 9.2987 |
0.3822 2.2434 -1.7824 3.1449 |
-10.5721 -9.5756 -4.7367 -8.6334 |
3.4375 -0.8669 6.7160 -5.4434 |
0.0950 0.0802 0.0717 0.1175 |
|
Excess Molar Volume |
||||||
|
303.15K 308.15K 313.15K 318.15K |
-1.3058 -1.4024 -1.4876 -1.6007 |
0.7426 0.6818 0.7518 0.8638 |
0.0737 0.2631 0.4300 0.5500 |
-0.5413 -0.4406 -0.5181 -0.7906 |
0.9325 0.3555 -0.2214 -1.3322 |
0.0072 0.0052 0.0070 0.0168 |
|
Excess Int. Mole Free Length |
||||||
|
303.15K 308.15K 313.15K 318.15K |
-0.0907 -0.1021 -0.1099 -0.1118 |
0.1479 0.1349 0.1126 0.1391 |
0.0103 0.0389 -0.0227 0.0510 |
-0.1693 -0.1512 -0.0755 -0.1328 |
0.0472 -0.0190 0.0971 -0.0848 |
0.0015 0.0013 0.0011 0.0018 |
|
Deviation in Viscosity |
||||||
|
303.15K 308.15K 313.15K 318.15K |
2.4355 1.7053 1.6755 0.9522 |
-0.4243 -0.9368 -0.6835 -0.3125 |
0.7484 0.4909 -0.3652 0.6860 |
-0.1506 1.9457 1.5361 0.6023 |
-2.5559 -1.0406 0.3330 -1.4678 |
0.0300 0.0086 0.0108 0.0074 |
The variations of excess molar volume, inter molecular free length, deviation in adiabatic compressibility and viscosity with the mole fraction of OCP for the studied systems are given in figures 1.1 to 3.4 respectively.
Using Nomoto’s relation (UNR), impedance relation (UIR), ideal mixing relation (Uimx), Junjie’s relation (UJ), Rao’s specific velocity relation (UR) and Kudriavtsev relation (UK), the theoretical values of ultrasonic velocity were evaluated. The computed estimations of ratio in velocity (U2/U2imx) from measured estimations of ultrasonic velocity (U) are graphically shown.From the values of experimental and theoretical velocities the molecular interaction parameter (α) has been evaluated and discussed its variation with the composition mixture has been conferred in terms of molecular interactions. The validity of the theories were checked by calculating standard deviation and chi square test.
The experimentally measured velocities and percentages of deviation using various theories are represented in table 4. Average percentage deviations and Chi square test are results are also given in table 4.
Table 4: Percentage deviations, interaction parameters (α) alongwith average percentage deviation and Chi square test values for the systems OCP + Methyl Salicylate, OCP + Ethyl Salicylate and OCP + Benzyl Salicylate
|
X1 |
UEXP |
%UN |
%Uimp |
%UVDV |
%UJUN |
%URAO |
U2/U2imx |
α |
|
OCP + Methyl Salicylate |
||||||||
|
303.15K |
||||||||
|
0.0000 0.1231 0.2401 0.3514 0.4573 0.5583 0.6547 0.7468 0.8349 0.9192 1.0000 |
1389.5 1395.8 1405.2 1411.9 1417.8 1423.0 1417.5 1410.0 1400.0 1390.0 1382.0 |
0.0000 -0.5023 -1.2191 -1.7394 -2.2002 -2.6100 -2.2853 -1.8196 -1.1735 -0.5187 0.0000 |
0.0000 -0.5252 -1.2581 -1.7887 -2.2546 -2.6647 -2.3365 -1.8634 -1.2062 -0.5367 0.0000 |
0.0000 -0.6809 -1.5187 -2.1119 -2.6045 -3.0107 -2.6548 -2.1310 -1.4027 -0.6434 0.0000 |
0.0000 -0.5650 -1.3308 -1.8868 -2.3697 -2.7877 -2.4583 -1.9735 -1.2929 -0.5871 0.0000 |
0.0000 -1.0655 -2.0941 -2.8237 -3.3750 -3.7658 -3.3957 -2.8204 -1.9653 -1.0320 0.0000 |
1.0000 1.0138 1.0311 1.0436 1.0542 1.0630 1.0553 1.0440 1.0287 1.0130 1.0000 |
0.0000 0.0138 0.0311 0.0436 0.0542 0.0630 0.0553 0.0440 0.0287 0.0130 0.0000 |
|
|
σ χ2 |
-0.1434 3.7643 |
-0.1472 3.9509 |
-0.1714 5.2425 |
-0.1557 4.3857 |
-0.2299 8.9782 |
||
|
|
||||||||
|
308.15K |
||||||||
|
0.0000 0.1231 0.2401 0.3514 0.4573 0.5583 0.6547 0.7468 0.8349 0.9192 1.0000 |
1374.4 1383.0 1392.0 1397.2 1403.1 1407.1 1401.0 1393.0 1383.0 1373.5 1361.2 |
0.0000 -0.7127 -1.4459 -1.9045 -2.4095 -2.7802 -2.4515 -1.9876 -1.3769 -0.7946 0.0000 |
0.0000 -0.7527 -1.5141 -1.9909 -2.5047 -2.8760 -2.5413 -2.0644 -1.4343 -0.8262 0.0000 |
0.0000 -0.9116 -1.7798 -2.3203 -2.8609 -3.2283 -2.8651 -2.3365 -1.6340 -0.9346 0.0000 |
0.0000 -0.7690 -1.5462 -2.0368 -2.5613 -2.9391 -2.6062 -2.1249 -1.4833 -0.8554 0.0000 |
0.0000 -1.2377 -2.2836 -2.9262 -3.5272 -3.8835 -3.5070 -2.9366 -2.1182 -1.2528 0.0000 |
1.0000 1.0185 1.0366 1.0481 1.0598 1.0678 1.0599 1.0484 1.0335 1.0190 1.0000 |
0.0000 0.0185 0.0366 0.0481 0.0598 0.0678 0.0599 0.0484 0.0335 0.0190 0.0000 |
|
σ χ2 |
-0.1619 4.5115 |
-0.1686 4.8720 |
-0.1934 6.3228 |
-0.1730 5.1155 |
-0.2439 9.7624 |
|||
|
313.15K |
||||||||
|
0.0000 0.1231 0.2401 0.3514 0.4573 0.5583 0.6547 0.7468 0.8349 0.9192 1.0000 |
1358.0 1369.5 1379.0 1384.0 1390.0 1394.0 1388.8 1379.7 1369.5 1359.5 1345.2 |
0.0000 -0.9289 -1.7008 -2.1460 -2.6590 -3.0296 -2.7591 -2.2119 -1.5795 -0.9532 0.0000 |
0.0000 -0.9676 -1.7669 -2.2296 -2.7511 -3.1224 -2.8459 -2.2863 -1.6350 -0.9838 0.0000 |
0.0000 -1.1263 -2.0321 -2.5584 -3.1067 -3.4740 -3.1690 -2.5580 -1.8345 -1.0921 0.0000 |
0.0000 -0.9831 -1.7973 -2.2732 -2.8049 -3.1824 -2.9075 -2.3438 -1.6816 -1.0116 0.0000 |
0.0000 -1.4191 -2.4831 -3.1055 -3.6953 -4.0552 -3.7492 -3.0849 -2.2406 -1.3530 0.0000 |
1.0000 1.0229 1.0419 1.0532 1.0652 1.0733 1.0665 1.0532 1.0377 1.0222 1.0000 |
0.0000 0.0229 0.0419 0.0532 0.0652 0.0733 0.0665 0.0532 0.0377 0.0222 0.0000 |
|
σ χ2 |
-0.1838 5.6141 |
-0.1903 6.0015 |
-0.2151 7.5934 |
-0.1945 6.2567 |
-0.2600 10.8529 |
|||
|
318.15K |
||||||||
|
0.0000 0.1231 0.2401 0.3514 0.4573 0.5583 0.6547 0.7468 0.8349 0.9192 1.0000 |
1343.4 1355.8 1365.5 1371.3 1377.0 1381.1 1374.5 1365.0 1354.0 1341.0 1324.1 |
0.0000 -1.0509 -1.8904 -2.4427 -2.9846 -3.4116 -3.0888 -2.5577 -1.9121 -1.1100 0.0000 |
0.0000 -1.1090 -1.9894 -2.5678 -3.1225 -3.5505 -3.2189 -2.6691 -1.9953 -1.1560 0.0000 |
0.0000 -1.2728 -2.2626 -2.9059 -3.4876 -3.9110 -3.5501 -2.9474 -2.1995 -1.2670 0.0000 |
0.0000 -1.0990 -1.9758 -2.5550 -3.1132 -3.5460 -3.2192 -2.6734 -2.0015 -1.1611 0.0000 |
0.0000 -1.4743 -2.5920 -3.3438 -3.9756 -4.3559 -3.9738 -3.3313 -2.4755 -1.4153 0.0000 |
1.0000 1.0260 1.0468 1.0608 1.0736 1.0831 1.0750 1.0617 1.0455 1.0258 1.0000 |
0.0000 0.0260 0.0468 0.0608 0.0736 0.0831 0.0750 0.0617 0.0455 0.0258 0.0000 |
|
σ χ2 |
0.0485 0.3653 |
-0.0107 0.0178 |
-0.2491 9.1423 |
-0.0569 0.4964 |
0.0280 0.1234 |
|||
|
OCP + Ethyl Salicylate |
||||||||
|
303.15K |
||||||||
|
0.0000 0.1280 0.2482 0.3614 0.4682 0.5691 0.6645 0.7550 0.8408 0.9224 1.0000 |
1355.5 1365.9 1378.3 1389.5 1399.0 1406.9 1405.0 1402.5 1395.6 1387.7 1382.0 |
0.0000 -0.5860 -1.3028 -1.9183 -2.4016 -2.7640 -2.4423 -2.0736 -1.3892 -0.6217 0.0000 |
0.0000 -0.4845 -1.1303 -1.7012 -2.1628 -2.5238 -2.2179 -1.8820 -1.2460 -0.5426 0.0000 |
0.0000 -0.8253 -1.6962 -2.3976 -2.9119 -3.2607 -2.8920 -2.4454 -1.6581 -0.7656 0.0000 |
0.0000 -0.6738 -1.4613 -2.1299 -2.6480 -3.0260 -2.7011 -2.3067 -1.5728 -0.7286 0.0000 |
0.0000 -1.2666 -2.3728 -3.2411 -3.8320 -4.1709 -3.7812 -3.2632 -2.3184 -1.2092 0.0000 |
1.0000 1.0167 1.0348 1.0497 1.0609 1.0685 1.0604 1.0508 1.0340 1.0155 1.0000 |
0.0000 0.0167 0.0348 0.0497 0.0609 0.0685 0.0604 0.0508 0.0340 0.0155 0.0000 |
|
σ χ2 |
-0.1582 4.4380 |
-0.1415 3.6063 |
-0.1933 6.4493 |
-0.1765 5.4461 |
-0.2630 11.4117 |
|||
|
308.15K |
||||||||
|
0.0000 0.1280 0.2482 0.3614 0.4682 0.5691 0.6645 0.7550 0.8408 0.9224 1.0000 |
1338.6 1350.0 1362.0 1373.0 1382.0 1389.0 1387.0 1383.5 1378.0 1368.4 1361.2 |
0.0000 -0.6926 -1.4139 -2.0481 -2.5281 -2.8588 -2.5545 -2.1397 -1.5764 -0.7080 0.0000 |
0.0000 -0.6061 -1.2668 -1.8629 -2.3243 -2.6538 -2.3628 -1.9760 -1.4542 -0.6405 0.0000 |
0.0000 -0.9484 -1.8350 -2.5618 -3.0759 -3.3933 -3.0391 -2.5414 -1.8672 -0.8642 0.0000 |
0.0000 -0.7741 -1.5609 -2.2441 -2.7561 -3.1011 -2.7934 -2.3549 -1.7455 -0.8065 0.0000 |
0.0000 -1.3318 -2.4410 -3.3003 -3.8931 -4.2048 -3.8304 -3.2712 -2.4495 -1.2383 0.0000 |
1.0000 1.0192 1.0377 1.0533 1.0645 1.0715 1.0637 1.0528 1.0384 1.0175 1.0000 |
0.0000 0.0192 0.0377 0.0533 0.0645 0.0715 0.0637 0.0528 0.0384 0.0175 0.0000 |
|
σ χ2 |
-0.1688 4.8808 |
-0.1545 4.1315 |
-0.2066 7.1453 |
-0.1857 5.8480 |
-0.2683 11.6489 |
|||
|
313.15K |
||||||||
|
0.0000 0.1280 0.2482 0.3614 0.4682 0.5691 0.6645 0.7550 0.8408 0.9224 1.0000 |
1324.0 1336.0 1348.5 1359.5 1368.0 1374.6 1373.0 1369.0 1364.0 1353.7 1345.2 |
0.0000 -0.7539 -1.5280 -2.1771 -2.6352 -2.9505 -2.6817 -2.2380 -1.7161 -0.8000 0.0000 |
0.0000 -0.6727 -1.3900 -2.0033 -2.4439 -2.7580 -2.5019 -2.0843 -1.6015 -0.7365 0.0000 |
0.0000 -1.0152 -1.9582 -2.7021 -3.1955 -3.4975 -3.1779 -2.6497 -2.0143 -0.9602 0.0000 |
0.0000 -0.8319 -1.6685 -2.3643 -2.8528 -3.1816 -2.9095 -2.4430 -1.8771 -0.8937 0.0000 |
0.0000 -1.3583 -2.4995 -3.3668 -3.9191 -4.2194 -3.8931 -3.2941 -2.5093 -1.2719 0.0000 |
1.0000 1.0206 1.0403 1.0563 1.0671 1.0738 1.0667 1.0552 1.0415 1.0195 1.0000 |
0.0000 0.0206 0.0403 0.0563 0.0671 0.0738 0.0667 0.0552 0.0415 0.0195 0.0000 |
|
σ χ2 |
-0.1788 5.3432 |
-0.1654 4.6067 |
-0.2175 7.7518 |
-0.1950 6.3007 |
-0.2723 11.8226 |
|
||
|
318.15K |
||||||||
|
0.0000 0.1280 0.2482 0.3614 0.4682 0.5691 0.6645 0.7550 0.8408 0.9224 1.0000 |
1306.8 1324.0 1341.5 1357.0 1370.0 1380.5 1383.9 1384.0 1382.0 1376.0 1369.0 |
0.0000 -0.8749 -1.7402 -2.4303 -2.9185 -3.2127 -2.9974 -2.5408 -1.9247 -1.0087 0.0000 |
0.0000 -0.6330 -1.3302 -1.9153 -2.3530 -2.6447 -2.4680 -2.0893 -1.5885 -0.8230 0.0000 |
0.0000 -0.9581 -1.8710 -2.5819 -3.0714 -3.3530 -3.1159 -2.6318 -1.9850 -1.0380 0.0000 |
0.0000 -0.9951 -1.9574 -2.7208 -3.2578 -3.5749 -3.3559 -2.8653 -2.1811 -1.1588 0.0000 |
0.0000 -1.3290 -2.4856 -3.3752 -3.9471 -4.1850 -3.8971 -3.3176 -2.4838 -1.3083 0.0000 |
1.0000 1.0194 1.0385 1.0537 1.0644 1.0706 1.0654 1.0548 1.0409 1.0211 1.0000 |
0.0000 0.0194 0.0385 0.0537 0.0644 0.0706 0.0654 0.0548 0.0409 0.0211 0.0000 |
|
|
σ χ2 |
0.0485 0.3653 |
-0.0107 0.0178 |
-0.2491 9.1423 |
-0.0569 0.4964 |
0.0280 0.1234 |
|
|
|
OCP + Benzyle Salicylate |
||||||||
|
303.15K |
||||||||
|
0.0000 0.1692 0.3143 0.4400 0.5500 0.6470 0.7333 0.8105 0.8800 0.9429 1.0000 |
1498.4 1489.0 1484.0 1479.0 1474.0 1468.0 1451.0 1435.0 1415.0 1396.0 1382.0 |
0.0000 -0.1043 -0.5155 -0.9396 -1.3768 -1.7605 -1.4164 -1.1441 -0.5969 -0.1174 0.0000 |
0.0000 -0.8360 -1.7127 -2.3972 -2.9325 -3.2837 -2.8080 -2.3074 -1.4494 -0.5800 0.0000 |
0.0000 -3.8964 -6.1491 -7.2915 -7.7401 -7.6652 -6.5669 -5.2775 -3.5178 -1.6511 0.0000 |
0.0000 -0.1503 -0.5980 -1.0487 -1.5024 -1.8926 -1.5452 -1.2586 -0.6858 -0.1683 0.0000 |
0.0000 -2.7750 -4.8204 -6.1771 -6.9622 -7.2162 -6.4391 -5.4012 -3.7671 -1.9174 0.0000 |
1.0000 1.0827 1.1353 1.1635 1.1748 1.1729 1.1455 1.1145 1.0742 1.0339 1.0000 |
0.0000 0.0827 0.1353 0.1635 0.1748 0.1729 0.1455 0.1145 0.0742 0.0339 0.0000 |
|
|
σ χ2 |
-0.0807 1.4373 |
-0.1876 6.4921 |
-0.5309 45.5765 |
-0.0897 1.7219 |
-0.4822 37.7513 |
||
|
308.15K |
||||||||
|
0.0000 0.1692 0.3143 0.4400 0.5500 0.6470 0.7333 0.8105 0.8800 0.9429 1.0000 |
1485.9 1477.0 1470.0 1465.1 1458.3 1450.0 1434.4 1415.6 1398.1 1377.0 1361.2 |
0.0000 -0.1953 -0.5317 -1.0235 -1.4005 -1.6901 -1.4950 -1.0836 -0.7650 -0.1899 0.0000 |
0.0000 -0.9788 -1.8152 -2.5859 -3.0699 -3.3267 -2.9887 -2.3345 -1.6799 -0.6870 0.0000 |
0.0000 -4.1190 -6.3580 -7.5842 -7.9754 -7.7970 -6.8148 -5.3602 -3.7814 -1.7760 0.0000 |
0.0000 -0.2445 -0.6199 -1.1401 -1.5350 -1.8316 -1.6329 -1.2064 -0.8601 -0.2445 0.0000 |
0.0000 -2.8290 -4.8048 -6.2025 -6.9379 -7.1075 -6.4691 -5.3009 -3.8871 -1.9385 0.0000 |
1.0000 1.0878 1.1404 1.1709 1.1808 1.1763 1.1516 1.1165 1.0801 1.0365 1.0000 |
0.0000 0.0878 0.1404 0.1709 0.1808 0.1763 0.1516 0.1165 0.0801 0.0365 0.0000 |
|
|
σ χ2 |
-0.0848 1.4745 |
-0.1997 7.0969 |
-0.5513 48.1288 |
-0.0944 1.7859 |
-0.4821 37.1668 |
||
|
313.15K |
||||||||
|
0.0000 0.1692 0.3143 0.4400 0.5500 0.6470 0.7333 0.8105 0.8800 0.9429 1.0000 |
1471.8 1463.0 1456.7 1452.2 1444.1 1435.2 1419.5 1402.0 1383.6 1361.7 1345.2 |
0.0000 -0.2186 -0.6203 -1.1582 -1.4644 -1.7296 -1.5392 -1.2288 -0.8573 -0.2314 0.0000 |
0.0000 -1.0163 -1.9268 -2.7481 -3.1646 -3.3972 -3.0612 -2.5022 -1.7892 -0.7380 0.0000 |
0.0000 -4.1862 -6.5053 -7.7800 -8.1043 -7.8980 -6.9123 -5.5439 -3.9026 -1.8337 0.0000 |
0.0000 -0.2688 -0.7103 -1.2771 -1.6015 -1.8739 -1.6798 -1.3538 -0.9541 -0.2870 0.0000 |
0.0000 -2.8046 -4.8155 -6.2458 -6.8942 -7.0451 -6.4263 -5.3475 -3.8844 -1.9139 0.0000 |
1.0000 1.0893 1.1440 1.1758 1.1842 1.1789 1.1540 1.1208 1.0829 1.0377 1.0000 |
0.0000 0.0893 0.1440 0.1758 0.1842 0.1789 0.1540 0.1208 0.0829 0.0377 0.0000 |
|
|
σ χ2 |
-0.0917 1.6538 |
-0.2089 7.6162 |
-0.5638 49.612 |
-0.1015 1.9900 |
-0.4810 36.6387 |
||
|
318.15K |
||||||||
|
0.0000 0.1692 0.3143 0.4400 0.5500 0.6470 0.7333 0.8105 0.8800 0.9429 1.0000 |
1457.0 1447.0 1441.1 1432.5 1425.5 1415.2 1398.0 1381.1 1363.0 1342.5 1324.1 |
0.0000 -0.1828 -0.6613 -0.9671 -1.3974 -1.6132 -1.3580 -1.1286 -0.8173 -0.3310 0.0000 |
0.0000 -1.0224 -2.0358 -2.6445 -3.1896 -3.3725 -2.9653 -2.4727 -1.8006 -0.8649 0.0000 |
0.0000 -4.2667 -6.7072 -7.7821 -8.2206 -7.9548 -6.8861 -5.5655 -3.9474 -1.9761 0.0000 |
0.0000 -0.2372 -0.7586 -1.0960 -1.5459 -1.7694 -1.5103 -1.2639 -0.9220 -0.3910 0.0000 |
0.0000 -2.6986 -4.7680 -5.9998 -6.7790 -6.8489 -6.1451 -5.1493 -3.7461 -1.9156 0.0000 |
1.0000 1.0911 1.1490 1.1759 1.1872 1.1803 1.1534 1.1213 1.0839 1.0407 1.0000 |
0.0000 0.0911 0.1490 0.1759 0.1872 0.1803 0.1534 0.1213 0.0839 0.0407 0.0000 |
|
σ χ2 |
0.0485 0.3653 |
-0.0107 0.0178 |
-0.2491 9.1423 |
-0.0569 0.4964 |
0.0280 0.1234 |
|||
 |
Figure 1.1: Variation of excess molar volume with the mole fraction of OCP for OCP+MS system |
 |
Figure 1.2: Variation of Adiabatic Compressibility with the mole fraction of OCP for OCP+MS system |
 |
Figure 1.3: Variation of excess intermolecular free length LfE with mole fraction of OCP for OCP + MS system. |
 |
Figure 1.4: Variation of deviation in viscosity with the molefraction of OCP for OCP + MS system |
 |
Figure 1.5 : Variation of U2/U2imx with the mole fraction of OCP for OCP + MS system |
 |
Figure 2.1: Variation of excess molar volume with the molefraction of OCP for OCP + ES system. |
 |
Figure 2.2: Variation of deviation in adiabtic compressiblity with the mole fraction of OCP for OCP + ES system |
 |
Figure 2.3: Variation of excess intermolecular free length with the molfraction of OCP for OCP + ES system |
 |
Figure 2.4: Variation of deviation in viscosity with the mole fraction of OCP for OCP + ES system |
 |
Figure 2.5: Variation of U2/U2imx with the mole fraction of OCP for OCP + ES system |
 |
Figure 3.1: Variation of excess molar volume with the mole fraction of OCP for OCP + BS system. |
 |
Figure 3.2: Variation of deviation in adiabatic compressibility with the mole fraction |
 |
Figure 3.3: Variation of deviation in excess intermolecular free length with the mole fraction of OCP for OCP + BS system. |
 |
Figure 3.4: Variation of deviation in viscosity with the mole fraction of OCP for OCP + BS system. |
 |
Figure 3.5: Variation of U2/ U2imx with the mole fraction of OCP for OCP + BS system. |
Discussion
Salicylates are known to exist in self associated molecular forming inter molecular hydrogen bonding through oxygen and OH group in ortho position. The effect of hydrogen bonding on the molar volumes of polar liquid mixtures was studied by Boule26. He found that the formation of complexes is hydrogen bonding is accompanied by a decrease in volume which is characteristic of extent of bonding.
When salicylate are mixed with solvents like OCP, change in volume of mixing can be due to (i) depolymersation of self associated hydrogen bonded salicylates (ii) formation of new hydrogen bonds between salicylates and OCP (iii) specific solute solvent interactions between salicylate and OCP. The first factor leads to positive excess volumes and second and third factors contribute to negative. The net volume change depends on the relative contributions of these factors.
In the present case, the actual change in volume in the reported binary systems is attributed to the dominance of second and the third factors of the above as well as the difference in size and shape of the component molecules. The negative deviation in all the systems shows that these systems have specific interactions between unlike molecules through hydrogen bonding. The order of VE values is given below.
Benzyl salicylate < Ethyl Salicylate < Methyl Salicylate
Same results were reported earlier workers27. Several workers28-30 reported that negative excess volumes indicate strong interactions between the components of mixtures. The VE values become more and more negative31,32 as the temperature is increased from 303.15 K to 318.15 K. This indicates that as the temperature increases the inter hydrogen bond in the associated salicylates become weaker and dipole. Dipole interactions between the hetero molecules get increased leading to greater contraction in volume.
Another interesting parameter, which is a representative of structural adjustment in solution, is inter molecular free length. Valuation of Lf a function of composition and temperature for all the systems indicates that Lf decreases with increase in mole fractions of OCP. Though strong interactions between unlike molecules are involve, the very small change in Lf is attributed due to braking of inter hydrogen bonding in the salicylates followed by the specified interactions between the molecules. The excess Lf values for all the systems show negative deviations indicating strong interactions between the components.
The deviations for all this systems are positive at all the temperatures. The volume and magnitude of Dh depends on the combined effects of the factors like molecular and shape of the components in addition to inter molecular forces. In systems when all types of interactions are operating, the values of Dh will be determined by the predominant effect. The deviations in viscosities in the present case are all positive in the entire range of composition at all temperatures indicate that specific interaction between unlike molecules are predominant which is in agreement with the conclusions drawn from data of velocity and other related parameters.
It is responsible for the constraints and approximations integrated into these theories. All of the molecules are thought to be spherical in shape, which is not always true. In Nomoto ‘s theory, when mixing, it is believed that the volume does not change. Therefore, no interaction has been taken into account between the components of liquid mixtures. The assumption for the development of the optimal mixing relationship is that the ratio of ideal mixtures’ unique heats and volumes are also equal. Again, it does not take into account any molecular interaction. Similarly, as per the belief of the theory of the Collision Factor, the molecules are handled, which is not really the case, as real non-elastic substances. But the interaction between the molecules of the two liquids happens when two liquids are combined due to the presence of different kinds of forces, such as dispersion forces, charge transfer, hydrogen bonding, dipole-dipole and dipole-induced dipole interactions. Therefore, the variations found in the velocity values measured using different models from the experimental values suggest that there is a molecular interaction between the different molecules in the liquid mixture. The computed estimations of ratio in velocity (U2 /U2imx) from measured estimations of ultrasonic velocity (U) are graphically shown in Fig 1.5, 2.5 and 3.5. The ratio (U2 /U2imx) is used as an important tool to measure the non-ideality in liquid mixtures, especially in these cases where the properties other than sound velocity are not known. The values of the (U2 /U2imx) are positive33 for the studied binary mixtures at all the different temperatures and over the entire composition range of OCP, which indicates dominance of associations over dispersion forces among the molecules of liquid mixture. Fig 1.5, 2.5 and 3.5 represent the variation of (U2 /U2imx) with mole fraction of OCP with branched alkanols. It is observed at 0.5 mole fraction systems show maximum positive deviation at all the temperatures, which infers the maximum hetero molecular associations at this mole fraction of OCP. The deviation of the ratio (U2 /U2imx) from unity is a direct measure of non ideality of the system as a consequence of association or dissociation.
The predicted ultrasonic velocities using various theories are reasonably close to the experimental values for and the three studied binary mixtures thus showing the validity of studied theoretical models for binary mixtures. These theories generally fail to predict accurately the ultrasonic velocities where strong interactions are supposed to exist and the average absolute percentage relative deviation is small in systems where the interactions are less or nil34.
The experimental values are close to the values computed using impedance relation in for OCP + Methyl salicylate, benzyl salicylate systems, where in the case of OCP + ethyl salicylate Nomoto’s theory is best suited. The deviation of the ratio (U2 /U2imx) from unity is positive which indicates strong interaction between the consecutive molecules35.
References
- K. N. Gayatri Devi, N.V.N.B. Srinivasa Rao , D. Ramachandran , V. Nagalakshmi , and P. Sunila Rani, Rasayan J of Chemistry, 15(1), 292(2022).
- Djazia Belhadj, Indra Bahadur, Amina Negadi, Natalia Muñoz-Rujas, Eduardo Montero and Latifa Negadi, J . Chem. Eng. Data 65(11), 5192(2020).
- D. Chinnarao, Ch . V Padmarao, K. Raja, M. Srilatha and B. Venkateswara Rao, IJERT, 9(4), 2020, DOI : 10.17577/IJERTV9IS040093
- Vipin Bihari Yadav, K.M. Singh, Juleshwar Prasad Kushwaha, Bulletin of Pure and Applied Sciences, 39 D (Physics)(2), (230)2020.
- Seema Agarwal, Dhirendra Kumar Sharma, Open Journal of Physical Chemistry, 11(3), 2021, DOI: 10.4236/ojpc.2021.113010
- F. F. Nobandegani, and A. Roeintan, American Journal of Mechanics and Applications, 7, 88, 2019. doi.org/10.11648/j.ajma.20190704.12
- A.K.Koneti, and S.Chintalapt Hindawi Publishing Corporation, London, 1, 2014. https://doi.org/10.1155/2014/343012
- R. D. Pawar, Sandip Patil and Govinda Waghulde, Rasayan Journal of Chemistry, 14(01), (584) 2014.
- A.Vishakha Telgote and Y.K. Meshram, IJCRT, 8(2), (1239) 2020.
- B.Uthayakumar, S .Ramadasse and G. Meenakshi, International Interdisciplinary Research Journal, III(VI), (243) 2013.
- G.R.Satyanarayana, D.Bala Karuna Kumar,K.Sujatha,G.Lakshmanarao and C.Rambabu, Journal of Molecular Liquids, 216, (256) 2016.
- D. Ubangara Mary and P.Neeraja, International Refereed Journal of Engineering and Science (IRJES) , 1(4), (54) 2012.
- Chandra Bhan Singh, Avanesh Kumar and Soran Singh, Rasayan J of chem, 5 (3), (420) 2012.
- P.S. Nikam, L.N. Shirsat and M. Hasan, Journal of Indian Chemical Society, 77, (244) 2000.
- Seyyed eh Narjes Mirheydari , Mohammad Barzegar-Jalali, Saeid Faraji, Hemayat Shekaari, Fleming Martinez and Abolghasem Jouyban, Physics and Chemistry of Liquids, (1) 2019 https://doi.org/10.1080/00319104.20 19.1625049.
- P.B. Sandhya Sri, Prof.C.Rambabu, D. B. Karuna Kumar, K.Rayapa Reddy, and G.Srinivasa Rao, International Journal of Advanced Research in Chemical Science, 2(2), (18) 2015.
- O.Nomoto, J Phys Soc, Japan, 4 ,(280) 1949, & 13,
- W. Van Dael and E.Vangeel, Pro Int. Conf on Cal & Therm.dyn, Warsa, 555, (1955) Jacobson B, Acta Chem Scandin, 1485, 6 ,(1952).
- M.Blandamer and D.Waddington, J Phys Chem, 74, (2569) 1970.
- Shipra Baluja and P.H.Parsania, Asian J Chem, 7, (417) 1995.
- V.D.Gokhale and N.N.Bhagavat, J Pure Appl Ultrason, 11, (21) 1989.
- Z.Junjie, J China Univ Sci Techn, 14, (298) 1984.
- S.G.M. Hussain, R. Kumar, V. Kannappan, I J of Innovative Research in Sc. Eng. & Tech. 5, (22) 2016).
- K.Pearson, Fundamentals of Mathematical Statistics, Eds., S.G. Gupta and V.K. Kapoor S. Chand and Company, New Delhi, India, 1973, 903.
- Jyh-Shing and Roger Jang, IEEE Transactions on Systems, Man and Cybernetics, 23(4), (665) 2014.
- P.Boule, Acid. Sci. Ser, 5, (268)1969.
- C.Jaya Prakash Raju, K.Rambabu, D.Ramachandran and C. Ramababu, Phy.Chem. 34, (51) 1997.
- G. V. Gangadhara Rao, S. Suriya Shihab, D. Das and Shaik. Babu and K.W. Chu, 13(4), (2123) 2020.
- A.J.Fort and W.R. Moore, Trans. Faraday. Soc, 61, (2102) 1965.
- S.S.Yadava, Y.Singh, Neetu Kushwaha, Global Journal of Molecular Sciences, 4 (2), (118) 2009.
- Ch. Udayalakshmi, Shaik Beebi, P.B. Sandhya Sri ,V.N.S.R. Venkateswararao, G. Lakshmana Rao, and C. Rambabu, Rasayan J of Chemistry, 13(7), (202) 2020.
- A.Nagarjuna, K.V.Yamini Kanth , G. Balaji Prakash and Debashis Das, Rasayan J of Chemistry, 12(4), (1774) 2019.
- G. Lakshmana Rao, G. R. Satyanarayana, V. N. S. R. Venkateswara Rao, K. A. K. Rajkumar and C. Rambabu, Journal of Chemical and Pharmaceutical Research, 7(6), (367), 2015.
- J.V.Srinivasu , K. Narendra , RanjanDey , B.SubbaRao, International Journal of Advanced Research in Physical Science (IJARPS) 3(6), (7), 2016.
- Ch. Udayalakshmi, K. A. K. Raj Kumar, V. N. S. R. Venkateswararao, P. B. Sandhyasri, G. R. Satyanarayana and C. Rambabu, Der Pharma Chemica, 8(5):(209) 2016

This work is licensed under a Creative Commons Attribution 4.0 International License.









