Synthesis, Spectral, Antimicrobial and In Vitro Antitumorous Studies of Copper Based Mulltidentate Thiosemicarbazones.
Department of Chemistry, Catholicate College, Pathanamthitta, Kerala 689642,India.
Corresponding Author E-mail: janeyginu@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390235
Article Received on : 30 Dec 2022
Article Accepted on :
Article Published : 11 Apr 2023
Reviewed by: Dr. Dojalisa Sahu
Second Review by: Dr. M Kavitha
Final Approval by: Dr. S.A. Iqbal
This is a report of the studies conducted on the synthesis, spectral, biological viz. antibacterial and antitumorous activities of some Copper complexes synthesized from a multidentate thiosemicarbazone ligand, HL2. This is based on a reaction between a secondary amine viz.N-phenyl piperazine and an N-substituted heterocyclic ketone viz. 2-Benzoylpyridine. The ligand exhibits a multidentate mode of coordination through N,N,S donor atoms in the copper complexes. The electron paramagnetic Resonance studies of the complexes(solution spectra) at LNT in DMF shows typical axial spectra with distinct g-values, gǁ& g┴indicating a slightly distorted four coordinated planar geometry. The biological studies viz. antibacterial and antitumorous studies suggest their use as competent antibacterial and antitumor agents.
KEYWORDS:Antitumorous studies; Copper complexes; EPR spectra; g-values; multidentate
Download this article as:| Copy the following to cite this article: Mathew J. M, Jacob S. Synthesis, Spectral, Antimicrobial and In Vitro Antitumorous Studies of Copper Based Mulltidentate Thiosemicarbazones. Orient J Chem 2023;39(2). |
| Copy the following to cite this URL: Mathew J. M, Jacob S. Synthesis, Spectral, Antimicrobial and In Vitro Antitumorous Studies of Copper Based Mulltidentate Thiosemicarbazones. Orient J Chem 2023;39(2). Available from: https://bit.ly/3KtZO8A |
Introduction
The metal complexes derived from the thiosemicarbazone ligands has gained large focus and become a major subject of research for many chemists. Their remarkable chelating ability bonding through sulphur and azomethine nitrogen atoms with transition elements belonging to first row has attracted many researchers.1-3The most striking biological properties include antitumorous, antioxidant, antibacterial, antifungal, anticancerogenic, besides showing insulinmimeticeffects.4-12 thiosemicarbazones exist in thione and thienol forms both in solution and solid state, most strikingly thione form stays in solution phase.
The biological activity of thiosemicarbazones with heterocyclic bases is proven to exhibit a N,N,S tridentate planar system of coordination enhancing the biological activity.13,14 The biological activity is rather well influenced by the point of attachment of thiosemicarbazone moiety with the heterocyclic ring system. The highest activity arises when it gets attached through the 2nd position and subsequently diminishes towards to positions 3rd or 4th. This work involves the synthesis, characterization, antibacterial and short term antitumorous properties of the copper complexes.Obtained from the thiosemicarbazone ligand, HL2.
Materials and Methods
Materials
Analytical grade pure chemicals and solvents were used in this study withoutfurtherpurification. The copper salts used were Cu(NO3)2·xH2O, CuCl2.2H2O, CuSO4.5H2O, Cu(CO2CH3)2.H2O, besides NaN3 and KSCN. Elemental C,H,N analyses was done using Elementar Vario ELIII analyzer. FTIR spectra was recorded as KBr pellets in the range 4000-400 cm-1 on a Thermo Nicolet Avatar FTIR spectrometer. The UV-Vis spectra were recorded using Varian Carry5000UV-VIS-NIR spectrophotometer inthe range900-250 nm.1H-NMR spectra was recorded in CDCl3 solvent using TMS as internal reference inaBruker400AvanceIIIspectrometer.The ESR-JEOL Japan instrument was used to record solution phase EPRspectra(X-Band) in frozen DMF at LNT(77K).
Synthesis of thiosemicarbazone ligand(HL2)
The already reported procedure was used to prepare compounds4-methyl-4-phenyl-3-thiosemicarbazide and carboxymethyl-N-methyl-N-phenyl dithiocarbamate.15,16 Equimolar amounts of 4-methyl-4-phenyl-3-thiosemicarbazide (1g, 5.52 mmol) dissolved in 5 ml hot methanol along with2-benzoylpyridine (1.011g, 5.52 mmol) and N-phenyl piperazine (0.84 ml, 5.52 mmol) separately dissolved in 10 ml methanol (99.9%) were refluxed for 45 minutes at 50° C. The resulting solution was left to chill which yielded 40%(0.160 g)yellow shining microcrystals of HL2 . The product so obtained was filtered, washed and recrystallized using methanol and kept in dessicator with P4O10 to remove all the moisture content. The synthesis of the ligand is as shown;.
 |
Scheme 1: Synthesis of ligand, HL2 |
Synthetic route of copper complexes
The methanolic solutions of appropriate copper salts and ligand, HL2 were refluxed. Chloro, nitrato and sulphato complexes were synthesized by refluxing solutions of respective hydrated copper (II) salts and ligand in 1:1 molar ratio for three hours. The mixture was allowed for slow evaporation at room temperature. Dark green colored microcrystals of copper (II) complexes obtained were filtered, washed with waterand ether and dried over P4O10 in vacuo.
The azido and thiocyanato complexes were synthesized by refluxing equimolar mixtures of ligand, HL2 and copper (II) acetate salt first followed by the addition of equimolar amounts of methanolic solution of sodium azide and potassium thiocyanate, respectively to the hot mixture and refluxed for next 3 hours. The mixture was left to cool at room temperature that gave rise to dark green microcrystals of azido and thiocyanato copper complexes. The complexes were filtered, washed with ether and dried over P4O10 in vacuo.
Bio-Assay
Antibacterial studies
The antibacterial studies the ligand, HL2 and synthesized copper complexes were done using agar well diffusion technique using MHA (Mueller Hinton Agar) medium and streptomycin positive control at two different concentrations.
Antitumorous Studies
The antitumorous studies of the copper complexes were done by Trypan blue dye exclusion method on DLA (“Daltons lymphoma ascites”) tumour cells derived from tumour bearing mice at different concentrations.
Results and Discussion
The C,H,N results of the complexes are in good agreement with the anticipated values as per the empirical formula [M(L)X], where M=Cu(II), L= deprotonated ligand, HL2and X=Cl, NO3, N3, SCN and [M2(L)2X] where X=SO4.The low molar conductivity values of the copper (II) complexes at room temperature in 10-3 M solution of DMF indicates their non-electrolytic nature suggesting the absence of counter ions outside the coordination sphere.17The magnetic moment values of copper complexes in the range 1.7-2.2 B. Mare characteristic of a d9 planar system in all the synthesized complexes. Table 1. shows colour, stoichiometries, elemental C,H,N data and magnetic moments of the copper (II) complexes of ligand HL2.
Table 1: Colour, elemental analyses data and magnetic moments of ligand HL2 and its copper (II) complexes.
|
Compound |
Formulaea |
Colour |
Composition% Found /(Calcd) |
μb (BM) |
||
|
|
|
|
Carbon (%) |
Hydrogen (%) |
Nitrogen (%) |
|
|
HL2 |
C23H23N5S. |
Yellow |
67.88 (67.32) |
6.00 (5.85) |
17.70 (17.61) |
– |
|
[Cu(L2)Cl] |
CuC23H22N5SCl |
Green |
55.24 (55.30) |
4.13 (4.39) |
13.91 (14.06) |
1.55 |
|
[Cu(L2)(NO3)] |
CuC23H22N6SO3 |
Green |
52.42 (52.51) |
4.10 (4.19) |
16.23 (15.98) |
1.73 |
|
[Cu2 (L2)2(SO4)].H2O |
Cu2C46H46N10S3O5. |
Green |
53.12 (53.01) |
4.20 (4.41) |
13.40 (13.44) |
1.82 |
|
[Cu(L2)N3] |
CuC23H22N8S |
Green |
54.91 (54.60) |
4.50 (4.34) |
22.02 (22.15) |
1.93 |
|
[Cu(L2)(NCS)] |
Cu C24H22N6S2 |
Green |
55.70(55.22) |
4.41 (4.21) |
16.41 (16.10) |
1.96 |
a Empirical formulae b Magnetic Susceptibility
Spectral Characterization
Infrared spectral studies
The positions of bands due to ν(C=C) and ν(C=N) vibrational modes vary from those of the ligand spectrum constituting bands in the 1600-1350 cm-1region in infrared spectra. This could be because the newly formed (N=C) bond overlaps with the ν(C=N) bond. Consequently the complexes shift the azomethine group stretching ν(C=N)str, thiosemicarbazone moiety band at 1592cm-1 in the ligand HL2, showing coordination through azomethine nitrogen. The ν(N-N) band shift at 1014cm-1 of thiosemicarbazone moiety in complexes further confirms coordination through azomethine nitrogen. The bands due to thiolate sulphur stretching, ν(C=S)str at 1339 cm-1 and thiolate sulphur bending, δ(C=S) at 870 cm-1 in HL2 shifts to lower frequenciesattributingtocoordinationviathethiolatesulphur.17 This implies substantial electron-delocalisation and a change of bond order occuring during chelation. The coordination through pyridyl nitrogen is confirmed by planebending vibrations of pyridine ring,δpy (o.p)at650 cm-1infreeligandshifting tohigher frequencies.17-19 Bands at 1430cm-1 and 1297cm-1with a spacing of~130cm-1 corresponding to ν1 and ν4 nitrato group vibrational modes are seen in the nitrato complex. This confirms terminally N-coordinated monodentate nitrato group coordination in the synthesized complex.17,19
The spectrum of synthesized sulpato complex contains two moderate intensity regions with bands at 923cm-1 and463cm-1 due to ν1 and ν2 vibrational modes, correspondingly. The ν3vibrational mode splits into three weak bands at 1021cm-1,1112cm-1,1226cm-1. The spectrum fails to show bands at 500, 575,825cm-1indicating water molecules to be present as lattice water.22 These spectral results indicate that in the synthesized complex, sulpha to anion adopts a bridging bidentate form of coordination.18,22
The synthesized azido complex exhibits strongbandsat2043cm-1due to the coordinated azido groups asymmetric,νa (NNN) vibration, and a strong band at1386cm-1 from its symmetric,νs(NNN)vibration. The δ(N-N-N)azido group vibrational modes are represented by the medium band at 648cm-1. These spectral data suggests a non-linear Cu-(NNN)bond in the synthesized azido complex.18 The synthesized thiocyana to complex of ligand HL2 demonstrates a very strong band at 2095cm-1characteristic of ν(C=N) thiocyana to group vibrations.Thebandsat787cm-1and463cm-1maybeallocated toν(C=S)andν(NCS)vibrations, of the thiocyana to complex. These facts confirm the thiocyanate anion to be N-coordinated to the copper (II)ion.
Table.2. shows some of the most important tentative IR spectral assignments of the ligand and its complexes .
Table 2: Infrared spectral assignments (cm-1) of ligand, HL2 and its copper (II) complexes.
|
Compound |
ν(C=N) & ν(N=C) |
ν (N-N) |
ν (C=S) & δ(C=S) |
δpy (o.p) |
|
HL2 |
1593 m |
1014 w |
1339 m, 788 m |
650 w |
|
[Cu(L2)Cl] |
1598 m |
1123 w |
1305 m, 763 m |
698 w |
|
[Cu(L2)(NO3)] |
1597 m |
1122 w |
1299 m, 759 m |
695 w |
|
[Cu2(L2)2(SO4)].H2O |
1598 m |
1112 w |
1307 m, 784 m |
684 m |
|
[Cu(L2)N3] |
1597 m |
1112 w |
1304 m, 779 m |
699 w |
|
[Cu(L2)(NCS)] |
1599 m |
1122w |
1301 m, 787m |
689 w |
Electronic Spectral studies
The band at282 nm(35,460cm-1)resulting from the pyridyl ring π→π* transitions shows a red shift because of >C=S bond weakening and strengthening of the conjugation system. The band at 341 nm (29,325cm-1)caused by the pyridyl nitrogen n→π* transition in the free ligand, HL2changes to shorter wavelengths or displays a blueshift and significantly loses intensity in complexes, indicating that pyridyl nitrogen serves as a coordinating molecule.28 The synthesized copper complexes exhibits two ligand-metal charge transfer, at about 380 nm (26,000cm-1) & 430nm (23,000cm-1). The band at higher wave number may be assigned to sulphur to copper(II) viz. ligand to metal charge transfer, whereas the second intense band in the range can be due to the combination of sulphur and pyridyl nitrogen combined charge transfer from ligand to copper (II) in the synthesized complexes.29,30 The synthesized complexes exhibit d-d bands as weak shoulders in the in the 500-625nm (16,000-20,000 cm-1) range which is typical of a square planar geometry.28Table 3. shows the tentative electronic spectral assignments of the ligand HL2 and its copper complexes recorded in the region 200-800 nm.
Table 3: Electronic spectral assignments (nm) of ligand HL2 and its copper (II) complexes.
|
Complex |
d-d bands |
Charge-transfer bands |
n→π* |
π→π* |
|
HL2 |
– |
– |
341,439 (sh) |
282 |
|
[Cu(L2)Cl] |
570 (sh) |
383 (sh), 432 (sh) |
337 |
286 |
|
[Cu(L2) (NO3)] |
527 (sh) |
375 (sh), 432 (sh) |
332 |
289 |
|
[Cu2( L2)2(SO4)].H2O |
569 (sh) |
377 (sh), 421 (sh) |
335 |
288 |
|
[Cu(L2)N3] |
543 (sh) |
378 (sh), 432 (sh) |
332 |
283 |
|
[Cu(L2)(NCS)] |
615 (sh) |
376 (sh), 429 (sh) |
322 |
285 |
EPR Spectral studies
EPR spectra of the synthesized copper complexes were recorded in DMF at 77K, (LNT). The EPR spectra (axial type) of chloride, nitrate and azide complexes in DMF at 77K, (LNT) is shown in Fig 1 (a, b, c) correspondingly. The spectra represents a typical axial pattern with distinct g ║and g┴ values varying for each complex. The G-values less than 4 in the range 3.5-4, are evident of appreciable exchange interaction. The x-y synthesized copper (II) complexes show g║>g┴>2 values coherent to a dX2-Y2ground state characteristic of a planar geometry. The synthesized complexes exhibit orbital reduction variables K║ and K┴, that are consistent with the trend, K‖< K┴ indicating π-bonding. Both in-plane σ and π bonding are consistent with the bonding parameters that were assessed.24 Three of the four hyperfine lines in the g║ area of the chloro and nitrato complexes, of ligand HL2 are clearly visible, while the fourth hyperfine line overlaps with the g┴ component leading to the observation of five super-hyperfine lines in the perpendicular component. Four hyperfine lines as well as seven super-hyperfine splitting can be seen in the g║ region of the azido and thiocyanato complex because of the electron spin coupling with the nitrogen atoms nuclear spinning in the azide as well as thiocyanate anions which are coordinating to the copper (II) ions. The assessed bonding parameters agree with both the in-plane s and p bonding. 24-30 Table 4. displays the spin Hamiltonian and bonding characteristics of the synthesized copper complexes.
Table 4: Spin Hamiltonian and Bonding parameters of ligand HL2 and its copper complexes.
|
DMF Soln(77K) |
[Cu(L2)Cl] |
[Cu(L2) (NO3)] |
[Cu2(L2)2 (SO4)].H2O |
[Cu(L2)N3] |
[Cu(L2) (NCS)] |
|
g║ |
2.034 |
2.057 |
2.046 |
2.031 |
2.030 |
|
g┴ |
2.125 |
2.199 |
2.170 |
2.114 |
2.110 |
|
gav |
2.0783 |
2.1267 |
2.092 |
2.072 |
2.082 |
|
A║ (mT) |
20.30 |
13.6 |
16.2 |
19.0 |
17.1 |
|
A┴ (mT) |
2.87 |
18.8 |
2.50 |
2.69 |
2.62 |
|
G |
3.8392 |
3.6629 |
3.7517 |
3.9268 |
3.8881 |
|
α2 |
0.7337 |
0.6469 |
0.6822 |
0.6855 |
0.6733 |
|
β2 |
0.7453 |
1.0795 |
0.9491 |
0.7710 |
0.7674 |
|
γ2 |
0.761 |
1.128 |
0.9805 |
0.7783 |
0.7784 |
|
K║ |
0.547 |
0.6983 |
0.6472 |
0.5287 |
0.5167 |
|
K┴ |
0.5584 |
0.7297 |
0.6684 |
0.5335 |
0.5240 |
 |
Figure 1: EPR spectra of [Cu(L2)Cl], [Cu(L2)(NO3)], [Cu(L2)N3]complexes in DMF at 77K, LNT |
 |
Figure 2: Proposed structures of the synthesized copper (II) complexes |
Bio Assay
Antibacterial studies
The copper (II) complexes were tested for antibacterial property using Mueller Hinton Agar (MHA) medium and streptomycin as the positive control against two Gram positive and two Gram negative strains of bacteria eat two sample concentrations.31 The inhibition zones produced by complexes against selected bacterial strains are shown in Fig 3. Gram Positive and Gram negative strains of bacteria chosen were; S. aureus (ATCC25923), S.mutans (MTCC890), E.coli (ATCC25922) and P. aeruginosa (ATCC27853).These copper (II) complexes exhibit antibacterial activities superior to the ligand HL2 showing inhibition zones above 1.0 cm. The highest activities have been recorded by [Cu(L2)N3 and [Cu(L2)(NCS)] complexes, producing inhibition zones of 2.2 cm against S. aureus (1000μg/ml) and S. mutans (1000μg/ml), respectively. In view of the chelation action which lowers the polarity of the metal ion one may hypothesize a potential route of toxicity.32,33 In addition to chelation, metal ion participation in normal cell activity might be responsible for the increased toxicity of the metal complexes.34 The most ideal location for metal ion interactions are in the walls of the cells and membranes with a lipophilic character. A subsequent reduction in the polarity strengthens the chelate’s lipophilic character which facilitates the metal ion interaction with the lipid. This causes the permeability barrier of the cell to break down, disrupting regular cell activities. Because all of the structures induce a range of functional groups that might serve as metal binding agents, metal ions interacting with binding molecules.34 The addition of anion coordination and polar substituents further enhance antibacterial activity. Table 5 shows the antibacterial screening results of copper (II) complexes of ligand HL2 by agar well diffusion method.
Table 5: The antibacterial results (inhibition zone in cm’s) of copper complexes of ligand HL2 following agar-well diffusion method.
|
Complexes |
Inhibition Zone (in cm’s) |
|||||||
|
Bacterial strains |
||||||||
|
S. aureus |
S. mutans |
P. aeruginosa |
E.coli |
|||||
|
C (I) |
C(II) |
C (I) |
C(II) |
C (I) |
C(II) |
C (I) |
C(II) |
|
|
HL2 |
1.6 |
1.9 |
– |
1.1 |
– |
– |
– |
1.3 |
|
[Cu(L2)Cl] |
1.1 |
1.4 |
1.6 |
2.1 |
– |
1.3 |
– |
1.3 |
|
[Cu(L2)(NO3] |
1.7 |
2.0 |
1.7 |
2.1 |
1.0 |
1.4 |
– |
1.6 |
|
[Cu2(L2)2(SO4)].H2O |
1.7 |
2.1 |
1.3 |
1.9 |
– |
1.8 |
– |
1.4 |
|
[Cu(L2)N3] |
1.9 |
2.2 |
1.4 |
2.0 |
– |
1.1 |
– |
1.7 |
|
[Cu(L2)(NCS)] |
1.2 |
1.5 |
1.4 |
2.2 |
– |
1.0 |
– |
1.1 |
C (I)=250 μg/ml, C (II) = 1000μg/ml
 |
Figure 3: petriplates showing inhibition zones produced by synthesized copper(II) complexes |
Antitumorous studies
Using (DLA) tumor cells aspirated from the peritoneal cavity of tumour bearing mice copper (II) complexes were investigated forantitumorous studies following the trypan blue dye exclusion approach. The photographs of cytotoxic screening results of the complexes are shown in Fig 4(a),(b)&(c). The cytotoxicity findings show extremely high activities ranging from 6% to 100% with the highest activity of 100% recorded for [Cu(L2)NCS], complex at 200 μg/ml concentration. These results suggest their use as effective antitumorous agents. This could be due to the fact that whereas healthy live cells are unstained because the stain is blocked from entering the cell due to a tough cell membrane, tumor affected cells with compensated cell membrane integrity allow the entrance of the trypan blue dye and appear blue. Table 6. Shows the short term in vitro cytotoxicity results of the copper (II) complexes of HL2.
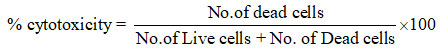
Table 6: The short term antitumorous screening results of the copper complexes of HL2
|
Complex concentration (μg/ml) |
% cytotoxicity of complexes |
||||
|
[Cu(L2)Cl] |
[Cu(L2)NO3] |
[Cu(L2)2SO4].H2O |
[Cu(L2)N3] |
[Cu(L2)NCS] |
|
|
10 |
20 |
6 |
15 |
20 |
40 |
|
20 |
48 |
12 |
20 |
38 |
52 |
|
50 |
58 |
22 |
26 |
50 |
75 |
|
100 |
70 |
36 |
35 |
63 |
88 |
|
200 |
82 |
58 |
46 |
84 |
100 |
 |
Figure 4: Photographs showing cytotoxic effects of the synthesized Copper complexes against tumor cells at 200(μg/ml) concentration |
Conclusions
In this analysis five complexes of thiosemicarbazone ligand (HL2) were well characterized after physicochemical and spectroscopic methods. The studies reveal a tridentate mode of coordination by ligand HL2 to the copper (II)ions in the complexes using thiolate sulphur, pyridyl nitrogen and azomethine nitrogen, holding the 1:1:1 metal: ligand: an ion coordination stoichiometry. Antibacterial screening results of copper complexes are superior to that of the ligand against selected strains of bacteriae suggesting their use as potent antibacterials. The short term in vitroantitumorous studies indicate their ability to show highly evolved activities against the tumour cells suggesting their use as competent antitumor agents.
Acknowledgements
The Sophisticated Analytical Instrumentation Facility, CUSAT and IIT Mumbai are collectively acknowledged for the physico-chemical data, spectral studies and for EPR studies. IIT Chennai for the magnetic susceptibility studies. The Center for molecular Biology and Applied Science Poojapura, Thiruvananthapuram Biogenix Research Centre, is deeply acknowledged for antimicrobial studies. Moreover Amala Cancer Research centre, Amalanagar, Thrissur, kerala is equally acknowledged for Short term in vitro cytotoxicity studies.
Conflicts of Interest
There are no conflict of interest.
References
- Polo-Cerón, D.;2019, Bioinorganic chemistry and applications,
CrossRef - Venugopal, R.; Sreejith S.S.; Kurup, M.R. P.; Polyhedron. 2018.
- Mathews N. A.; Kurup M.R.P.; Applied Organic Chemistry. 2020.
- MatesanzA. I.; Herrero J. M.;& Quiroga, A. G.; Current topics in medicinal chemistry, 2021, 21(1), 59-72.
CrossRef - Rajendran, N.; Kamatchi, N.; Periyasamy, A.;& Solomon, V.; Journal of Coordination Chemistry, 2020, 73(6), 969-985.
CrossRef - Seena, E.B.; Sithambaresan, M.; Vasudev, S.; Kurup, M. R.P. J. Chem. Sci. 2020,132, 149.
CrossRef - Damit, N. S. H. H.; Hamid, M. H. S. A.; Rahman, N. S. R. H. A.; Ilias, S. N. H. H.;& Keasberry, N. A.; Inorganica Chimica Acta, 2021,527, 120557.
CrossRef - Prajapati, N. P.;& Patel, H. D.;Synthetic Communications. 2019, 49 (21), 2767-2804.
- Haseloer, A.; Denkler, L. M.; Jordan, R.; Reimer, M.; Olthof, S.; Schmidt, I.; Klein, A Dalton Transactions.,2021, 50,12, 4311-4322.
CrossRef - Matesanz, A. I.; Herrero, J. M.; & Quiroga, A. G.; Current topics in medicinal chemistry., 2021, 21,1, 59-72.
CrossRef - Shakya, B.; Yadav, P. N.;Mini Reviews in Medicinal Chemistry, 2020,20, 8, 638-661.
CrossRef - Savir, S.; Liew, J. W. K.; Vythilingam, I.; Lim, Y. A. L.; Tan, C. H.; Sim, K. S.; Tan, K. W.; Journal of Molecular Structure, 2021, 1242, 130815.
CrossRef - Gu, S.; Yu, P.; Hu, J.; Liu, Y.; Li, Z.; Qian, Y.;Yang, F. European journal of medicinal chemistry., 2019, 164, 654-664.
CrossRef - Deng, J.; Yu, P.; Zhang, Z.; Wang, J.; Cai, J.; Wu, N.; Yang, F. European Journal of Medicinal Chemistry. 2018, 158, 442-452.
CrossRef - Klayman D.L; and Lin, A. J.; Org. Prep. Proc. Int.; 1981, 16, 79. J. Med. Chem., 44 (2009) 818.
- Scovill, J. P.; Phosphorus, Sulfur and Silicon and the Related Elements., 1991,60 (1-2), 15-19.
CrossRef - Geary, W. J.; Coordination Chemistry Reviews., 1971,7, 1, 81-122.
CrossRef - Nakamoto, K.Infrared and Raman Spectra of Coordination Compounds.3rd Ed.; John Wiley and Sons.
- Kumar, B.; and Kumar, A.; International Journal of Scientific Engineering and Applied Science,1,5, 20152395-3470.
- Sreekanth, A., & Kurup, M. R. P.; Polyhedron, 2003, 22, 25-26, 3321-3332.
CrossRef - A.B.P.Lever.;Inorg .Chem.,1964,4 1042.
CrossRef - Nakamoto, K.; Infrared and Raman Spectra of Inorganic and Coordination Compounds., Wiley Inter science, New York, 4thEd, (1986), p283.
- Stefov, V.; Petruševski, V. M.; & Šoptrajanov, B.; Journal of molecular structure.,1993., 293, 97-100.
CrossRef - B.J Hathaway in Comprehensive Coordination Chemistry (Wilkinson, RD Gillard, JA Mc Cleverty)., Pergamon, Oxford, 1987, Vol.2, Ch. 15.5
- Kivelson, D.;& Neiman, R. The Journal of Chemical Physics, 1961, 35, 1, 149-155.
CrossRef - Huheey, J. E.; Keiter, E. A.; Keiter, R. L.;& Medhi, O. K.; Inorganic chemistry: principles of structure and reactivity.,2006, Pearson Education India.
- Drago, R.S.; Physical Methods in Inorganic Chemistry, Reinhold Publishing Corporation., New York. 1979, 4thEdition.
- Reena, T. A.; Seena, E. B.; & Kurup, M. P;. Polyhedron., 2008, 27(6), 1825-1831.
CrossRef - Latheef, L.; & Kurup, M. R. P.; Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy., 2008, 70 (1), 86-93.
CrossRef - Seena, E. B.;& Kurup, M. R. P.; Polyhedron.; 2007, 26 (4), 829-836.
CrossRef - National Committee for Clinical Laboratory Standards. (1993a).,(NCCLS,1993)2006, 89-98.
- Thangadurai, T. D.;& Natarajan, K.; Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry., 2001,31 (4), 549-567.
CrossRef - El-Sawaf, A. K.; El-Essawy, F.; Nassar, A. A.; & El-Samanody, E. S. A.; Journal of Molecular Structure., 2018,1157, 381-394.
CrossRef - Netalkar, P. P.; Netalkar, S. P.;& Revankar, V. K.; Polyhedron., 2015, 100, 215-222.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










