Adamantane-pyrido[2,3-d]Pyrimidine Derivatives; Synthesis, Characterization and Investigation of Antimicrobial Study
Manishkumar Jinabhai Tank , Navinkumar A. Kucha
, Navinkumar A. Kucha , Chirag G. Naik
, Chirag G. Naik , Tina R. Barot
, Tina R. Barot
, G. M. Malik1*
Department of chemistry, Navyug science college, rander road surat, Gujarat, India.
Corresponding Author E-mail: gmmalik2010@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390219
Article Received on : 05 Dec 2022
Article Accepted on : 02 Apr 2023
Article Published : 14 Apr 2023
Reviewed by: Dr. Prabhat Kumar Baroliya
Second Review by: Dr. Santhi R
Final Approval by: Dr. Ganesamoorthy Thirunarayanan
Target molecules based on Adamantane-pyrido[2,3-d]pyrimidine derivatives were prepared. Adamantane-pyrido[2,3-d]pyrimidine series using N-(hydroxyadamantan-1-yl)-5-(2,4-substitutedphenyl)-2-Methyl-4-Oxo-7-(2-oxo-2H-Chromen-3-yl)pyrido[2,3-d]Pyrimidine-3(4H)carboxamide (6a-j) was synthesized by reaction between 3-(2-chloroacetyl)-5-(2,4-substitutedphenyl)-2-Methyl-7-(2-Oxo-2H-Chromen-3-yl) pyrido[2,3-d]pyrimidin-4(3H)-one (5a-j) and 3-aminoadamantan-1-ol. These derivatives of Adamantane-pyrido[2,3-d]Pyrimidine were investigated in vitro for their biological characteristics against the strains which were isolated clinically and confirmation of their structure was done by FTIR, 1H-NMR, 13C NMR and LCMS. The newly synthesized derivatives gave promising antimicrobial activity.
KEYWORDS:Adamantane, pyrido[2,3-d]pyrimidine; antimicrobial, antifungal; 3-aminoadamantan-1-ol; Chromene; N-hydroxy adamantan-1-yl; pyrido[2,3-d]pyrimidine; Pyrimidine
Download this article as:| Copy the following to cite this article: Tank M. J, Kucha N. A, Naik C. G, Barot T. R, Malik G. M. Adamantane-pyrido[2,3-d]Pyrimidine Derivatives; Synthesis, Characterization and Investigation of Antimicrobial Study. Orient J Chem 2023;39(1). |
| Copy the following to cite this URL: Tank M. J, Kucha N. A, Naik C. G, Barot T. R, Malik G. M. Adamantane-pyrido[2,3-d]Pyrimidine Derivatives; Synthesis, Characterization and Investigation of Antimicrobial Study. Orient J Chem 2023;39(1). Available from: https://bit.ly/3zUHqRo |
Introduction
The research on biochemical importance of pyrimidines and pyrimidine derivatives have centered great importance because of the pyrimidines represent the main backbone in alkaloids and nucleic bases as well as their interesting powerful biological activities. Pyrimidine derivatives contain diversified applications as pharmaceuticals and occupy a unique place in heterocyclic and medicinal chemistry also1-3. Combination of coumarin derivatives and pyrimidine derivatives has received considerable attention by researchers because of possessing so many biological important application and pharmacological activities4-7. Various pyrimidine derivatives show very broad range of biological activities viz. antimicrobial activity8, anti-inflammatory9, anticancer10, antiviral11, antitubercular12, antihypertensive13-14, anticonvulsant15, H1-antihistamines16, 4-phosphodiester inhibitors17-18 and antimalarial19-20.
Materials and Methods
The synthesis was carried out using A R Grade reagents and solvents and were used without further purification. Open capillary method was used to take melting points and are uncorrected. TLC (thin layer chromatography) was used check the progress of reactions using silica gel plates GF254 (E. Merck). The solvent system comprised of methanol and toluene; the chromatograms visualized using source of UV light (254nm). FTIR spectra were recorded making use of KBr on pallets Perkin Elmer 1600 FTIR. 1H NMR and 13C NMR spectra were obtained using Bruker 500 MHz, DMSO-d6 as the solvent and TMS (tetra methyl silane) as internal standard. LCMS was used to carry out LC-MS.
Synthesis of 3-Acetyl-2H-chromen-2-one (1)
0.01 mole Salicylaldehyde and 0.01 mole EAA (Ethyl Acetoacetate) was mixed in 15mL in ethyl alcohol. 2mL DEA (diethyl aniline) was added in this mixture with continuous stirring at RT for about 2 hrs which yielded solid. The solid was filtered, recrystallized using ethyl alcohol as solvent. Yield; 91 %, MP 113-115º C.
Preparation of various substituted chalcone derivatives (2a-k)
Base catalyzed Claisen-Schmidt condensation reaction was used to synthesize various chalcone derivatives of the appropriate substituted aldehydes and substituted acetophenone by reported literature method21.
3-Acetyl-2H-chromen-2-one (0.01mole) (1) and substituted benzaldehyde (0.01mole) dissolved in 10 mL ethanol in RBF using a magnetic stirrer. Water bath was used to maintain the temperature of reaction at 20-25º C. 1 gm NaOH in 10 mL dist. H2O was taken and this NaOH solution was drop wise added into to the reaction mixture for 30 minutes with continuous stirring. On completion of addition, solution was stirred further for 4-5 hrs and kept at RTfor 12 hrs. The final solution was dumped into chilled H2O & neutralized using 0.1-0.2N HCl whereby solid obtained. The product was filtered & then dried in air. The crude was recrystallized by rectified spirit. Further purification was done by used ethyl acetate and n-hexane.
Preparation of various derivatives of 2-Amino-4-(2,4-substitutedphenyl)-6-(2-oxo-2H-chromen-3-yl) nicotinonitrile (3a-k)
Chalcone derivatives (2a-k) (0.01mole), malononitrile (0.01mole) and anhydrous ammonium acetate (0.02mole) were taken in RBF and dissolved in 20 mL absolute ethanol solvent. It was heated under reflux condition for 7-8 hours. Completion of reaction was confirmed by TLC. This reaction mixture was then cooled down to the RT. As solution attained RT, solid was formed which was filtered, then was washed with distilled water till free from impurities dried and ethanol was used for recrystallized to obtains compounds (3a-k)22.
Preparation of various derivatives of 5-(2,4-substitutedphenyl)-2-Methyl-7-(2-oxo-2H–
chromen-3-yl) Pyrido[2,3-d] Pyrimidin-4(3H)-one (4a-k)
The mixture of compound (3a-k) (0.01mole) and excess of glacial CH3COOH (20mL) was heated maintaining reflux condition for 7-8 hours. Glacial CH3COOH was self-solvent. On completion of the reaction, solution obtained was cooled down to RT. The resultant solution was added in to chilled H2O. The solid formed was filtered, then washed with cold dist. H2O several times, dried & recrystallization from dioxane yielded compounds (4a-k).
Preparation of various derivatives of 3-(2-Chloroacetyl)-5-(2,4-substitutedphenyl)-2-Methyl-7-(2-Oxo-2H– Chromen-3-yl) Pyrido[2,3-d]Pyrimidin-4(3H)-one (5a-k)
Sodium acetate was dissolved in 20mL glacial acetic acid in RBF. Compound (4a-k) (0.01mole) was dissolved in this mixture and was cooled 0-5º C. Chloroacetyl chloride (0.02mL) was added in this mixture at 0-5º C during 1 Hr. After the completion of addition, resultant solution was stirred for 30 mins. The temperature was raised to 80º C for heating up to 1.5 Hrs. & then was stirred at R.T. This solution was dumped into chilled H2O, the solid thus formed was filtered, washed using chilled H2O several times, dried, recrystallized from acetic acid to give compounds (5a-k).
Preparation of various derivatives N-(Hydroxyadamantan-1-yl)-5-(2,4-substituted phenyl)-2-Methyl-4-oxo-7-(2-Oxo-2H-Chromen-3-yl)Pyrido[2,3-d]Pyrimidine-3(4H) Carboxamide (6a-k)
Compound (5a-k) (0.01mole) was dissolved in 20mL DMF. Slow heating was started and mixture of 3-Aminoadamantan-1-ol (0.01mole) and K2CO3 (0.012mole) was mixed slot wise. On completion of addition, the resultant solution was refluxed for 5-6 Hrs. TLC was used to check completion of reaction. The resultant solution was allowed to cool to RT, then dumped into ice, the solid formed was filtered, then washed with dist. H2O several times, dried, finally recrystallized using ethanol to yield compounds (6a-k).
Reaction scheme
 |
Scheme 1 Click here to View Scheme |
Table 1: Physical properties of synthesized Adamantane-pyrido[2,3-d]pyrimidine derivatives (TT1 to TT11)
|
No |
Sample |
Sample Code |
Molecular Formula |
Substituent |
Melting Point (°C) |
|
|
R1 |
R2 |
|||||
|
1 |
6a |
TT1 |
C35H32N4O6 |
-H |
-OCH3 |
278-280º C |
|
2 |
6b |
TT2 |
C34H29ClN4O5 |
-H |
-Cl |
285-287º C |
|
3 |
6c |
TT3 |
C36H35N5O5 |
-H |
-N(CH3)2 |
272-274º C |
|
4 |
6d |
TT4 |
C34H30N4O6 |
-H |
-OH |
286-288º C |
|
5 |
6e |
TT5 |
C34H29N5O7 |
-H |
-NO2 |
275-276º C |
|
6 |
6f |
TT6 |
C34H29BrN4O5 |
-H |
-Br |
266-268º C |
|
7 |
6g |
TT7 |
C34H30N4O6 |
-OH |
-H |
258-260º C |
|
8 |
6h |
TT8 |
C35H32N4O6 |
-H |
-CH3 |
288-290º C |
|
9 |
6i |
TT9 |
C34H28Cl2N4O5 |
-Cl |
-Cl |
280-282º C |
|
10 |
6j |
TT10 |
C35H32N4O6 |
-CH3 |
-H |
272-273º C |
|
11 |
6k |
TT11 |
C34H29BrN4O5 |
-Br |
-H |
255-257º C |
Table 2: Elementary analysis data of Adamantane-pyrido[2,3-d]pyrimidine derivatives (TT1 to TT11).
|
No |
Sample Code |
Elementary Analysis |
|||||||||||
|
Calculated (%) |
Found (%) |
||||||||||||
|
C |
H |
O |
N |
Cl |
Br |
C |
H |
N |
O |
Cl |
Br |
||
|
1 |
TT1 |
69.52 |
5.33 |
15.88 |
9.27 |
– |
– |
69.48 |
5.27 |
15.86 |
9.23 |
– |
– |
|
2 |
TT2 |
67.05 |
4.80 |
13.13 |
9.20 |
5.82 |
– |
67.00 |
4.77 |
13.09 |
9.18 |
5.79 |
– |
|
3 |
TT3 |
70.00 |
5.71 |
12.95 |
11.34 |
– |
– |
69.98 |
5.67 |
12.89 |
11.30 |
– |
– |
|
4 |
TT4 |
69.14 |
5.12 |
16.25 |
9.49 |
– |
– |
69.10 |
5.10 |
16.20 |
9.42 |
– |
– |
|
5 |
TT5 |
65.91 |
4.72 |
18.07 |
11.30 |
– |
– |
65.89 |
4.69 |
17.98 |
11.27 |
– |
– |
|
6 |
TT6 |
62.49 |
4.47 |
12.24 |
8.57 |
– |
12.23 |
62.47 |
4.44 |
12.22 |
8.55 |
– |
12.19 |
|
7 |
TT7 |
69.14 |
5.12 |
16.25 |
9.49 |
– |
– |
69.08 |
5.08 |
16.14 |
9.44 |
– |
– |
|
8 |
TT8 |
69.52 |
5.33 |
15.88 |
9.27 |
– |
– |
69.49 |
5.31 |
15.87 |
9.25 |
– |
– |
|
9 |
TT9 |
63.46 |
4.39 |
12.43 |
8.71 |
11.02 |
– |
63.44 |
4.31 |
12.35 |
8.64 |
10.88 |
– |
|
10 |
TT10 |
69.52 |
5.33 |
15.88 |
9.27 |
– |
– |
69.47 |
5.22 |
15.81 |
9.22 |
– |
– |
|
11 |
TT11 |
62.49 |
4.47 |
12.24 |
8.57 |
– |
12.23 |
62.40 |
4.42 |
12.21 |
8.51 |
– |
12.15 |
Table 3: Antibacterial activity of Adamantane-pyrido[2,3-d]pyrimidine derivatives (TT1 to TT11).
|
Antibacterial Activity |
|||||
|
Minimum Inhibition Concentration |
|||||
|
Sample |
Sample Code |
E. Coli MTCC443 |
P. Aeruginosa MTCC1688 |
S. Aureus MTCC96 |
S. Pyogenes MTCC442 |
|
6a |
TT1 |
50 |
62.5 |
100 |
62.5 |
|
6b |
TT2 |
100 |
100 |
200 |
62.5 |
|
6c |
TT3 |
62.5 |
50 |
200 |
100 |
|
6d |
TT4 |
100 |
62.5 |
100 |
200 |
|
6e |
TT5 |
100 |
200 |
200 |
200 |
|
6f |
TT6 |
50 |
100 |
250 |
200 |
|
6g |
TT7 |
100 |
200 |
250 |
200 |
|
6h |
TT8 |
50 |
62.5 |
200 |
62.5 |
|
6i |
TT9 |
50 |
100 |
50 |
100 |
|
6j |
TT10 |
62.5 |
100 |
100 |
100 |
|
6k |
TT11 |
100 |
200 |
100 |
200 |
|
Ampicillin |
100 |
100 |
250 |
100 |
|
|
Chloramphenicol |
50 |
50 |
50 |
50 |
|
|
Norfloxacin |
10 |
10 |
10 |
10 |
|
|
Ciprofloxacin |
25 |
25 |
50 |
50 |
|
Table 4: Antifungal activity and antitubercular of synthesized Adamantane-pyrido[2,3-d]pyrimidine derivatives (TT1 to TT11).
|
Antifungal activity & Antitubercular Activity |
|||||
|
Minimum Inhibition Concentration |
|||||
|
Sample |
Sample Code |
C. Albicans MTCC227 |
A. Niger MTCC282 |
A. Clavatus MTcc1323 |
H37RV MIC µg/mL |
|
6a |
TT1 |
100 |
100 |
50 |
100 |
|
6b |
TT2 |
50 |
62.5 |
100 |
62.5 |
|
6c |
TT3 |
200 |
100 |
200 |
100 |
|
6d |
TT4 |
100 |
100 |
250 |
500 |
|
6e |
TT5 |
62.5 |
50 |
62.5 |
100 |
|
6f |
TT6 |
50 |
100 |
100 |
200 |
|
6g |
TT7 |
100 |
100 |
200 |
62.5 |
|
6h |
TT8 |
62.5 |
100 |
50 |
100 |
|
6i |
TT9 |
200 |
200 |
200 |
500 |
|
6j |
TT10 |
200 |
250 |
100 |
100 |
|
6k |
TT11 |
100 |
100 |
100 |
62.5 |
|
Nystatin |
100 |
100 |
100 |
– |
|
|
Griseofulvin |
500 |
100 |
100 |
– |
|
|
Rifampicin |
– |
– |
– |
40 |
|
|
Isoniazid |
– |
– |
– |
0.2 |
|
Table 5: Antimalarial activity of synthesized Adamantane-pyrido[2,3-d]pyrimidine derivatives (TT1 to TT11).
|
Antimalarial Activity |
||
|
Minimum Inhibition Concentration |
||
|
Sample |
Sample Code |
Mean Values |
|
6a |
TT1 |
0.88µg/mL |
|
6b |
TT2 |
0.25µg/mL |
|
6c |
TT3 |
1.01µg/mL |
|
6d |
TT4 |
0.98µg/mL |
|
6e |
TT5 |
0.74µg/mL |
|
6f |
TT6 |
0.42µg/mL |
|
6g |
TT7 |
0.35µg/mL |
|
6h |
TT8 |
0.46µg/mL |
|
6i |
TT9 |
0.31µg/mL |
|
6j |
TT10 |
0.52µg/mL |
|
6k |
TT11 |
0.23µg/mL |
|
Chloroquine |
0.020 µg/mL |
|
|
Quinine |
0.268 µg/mL |
|
1H NMR spectral data of Adamantane-pyrido[2,3-d]pyrimidine derivatives
 |
Figure:1 1H NMR spectral data of TT1 |
1H NMR data (500 MHz, DMSO – d6) δ
1.57-2.24(m, 14H of adamantane), 3.47 (s, OH group of admentanol), 7.35 (s, 1H of NH group of adamantane), 3.07 (s, 3H of -CH3 of pyrimidine), 3.90 (s, 3H of CH3 of methoxy group), 6.94-8.59 (m, 10H of Aromatic group).
 |
Figure 2: 1H NMR spectral data of TT3 |
1H NMR data (500 MHz, DMSO – d6) δ
1.55-2.24(m, 14H of adamantane), 3.43 (s, OH group of admentanol), 7.41 (s, 1H of NH group of admantan), 3.09 (s, 3H of CH3 of pyrimidine), 3.92 (s, 6H of N(CH3)2 group), 6.75-8.51 (m, 10H of Aromatic group).
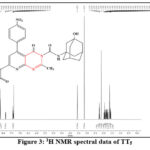 |
Figure 3: 1H NMR spectral data of TT5 |
1H NMR data (500 MHz, DMSO – d6) δ
1.55-2.24 (m, 14H of adamantane), 3.55 (s, OH group of admentanol), 7.41 (s, 1H of NH group of admantan), 3.13 (s, 3H of CH3 of pyrimidine), 7.35-8.60 (m, 10H of Aromatic group).
 |
Figure 4: 1H NMR spectral data of TT8 |
1H NMR data (500 MHz, DMSO – d6) δ
1.55-2.37 (m, 14H of adamantane), 3.55 (s, OH group of admentanol), 7.35 (s, 1H of NH group of admantan), 3.13 (s, 3H of CH3 of pyrimidine), 7.10-8.60 (m, 10H of Aromatic group), 2.73 (s, 3H of CH3 group).
13C NMR spectral data of Adamantane- pyrido[2,3-d]pyrimidine derivatives
 |
Figure 5: 13C NMR spectral data of TT1 |
Compound-TT2
22.40, 29.71, 35.98, 43.07, 44.40, 47.56, 50.50, 55.35, 68.80, 113.88, 114.98, 119.95, 120.24, 122.36, 125.51, 127.56, 129.59, 131.33, 132.68, 143.60, 145.30, 148.78, 152.96, 153.90, 154.04, 158.73, 159.49, 163.34, 164.02
IR Spectra of Adamantane-pyrido[2,3-d]pyrimidine derivatives
 |
Figure 6: IR Spectra of TT1 |
Compound-TT1: IR (KBr, cm-1)
ν = C-H 1271, N-H 1526 secondary amine, O-H 3924, C-H 2855 of OCH3, C-H 3413, C-Br 738, C-H 2922 of methyl group.
 |
Figure 7: IR Spectra of TT3 |
Compound-TT3: IR (KBr, cm-1)
ν = C-H 1275, N-H 1541 secondary amine, O-H 3926, C-H 3413, C-Br 738, C-H 2925 of methyl group. C-H 2925 of N(CH3)2
LCMS Spectra of Adamantane- pyrido[2,3-d]pyrimidine derivatives
 |
Figure 8: LCMS Spectra of TT1 and TT3 |
 |
Graph 1: Antimicrobial activity of Adamantane-pyrido[2,3-d]pyrimidine derivatives (TT1 to TT11) Click here to View Graph |
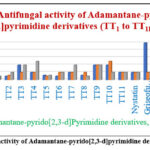 |
Graph 2: Antifungal activity of Adamantane-pyrido[2,3-d]pyrimidine derivatives (TT1 to TT11). |
 |
Graph 3: Anti Tubercular activity of Adamantane-pyrido[2,3-d]pyrimidine derivatives (TT1 to TT11) |
Results and discussion
3-Acetyl-2H-Chromen-2-one resultedby the reaction of Ethylacetoacetate (EAA) & Salicylaldehyde. (DEA) Diethyl Aniline then was mixed with continuous stirring dropwise at RT to obtain solid. Various chalcone compounds were prepared using Claisen-Schmidt (base catalyzed) condensation reaction of selected substituted aldehyde and substituted acetophenone by known literature method19. Substituted benzaldehyde and 3-Acetyl-2H-Chromen-2-one was mixed in ethanol using magnetic stirrer. Water bath was used to maintain the reaction temperature between 20-25º C on the magnetic stirrer. 1 gm NaOH was added to 10 mL distilled water and the resulted aqueous NaOH solution was dropwise added into the to the reaction mixture and when addition was completed this solution was stirred further for 4-5 Hrs and kept for 12 Hrs. The mixture was made neutral with 0.1 – 0.2N HCl till the solidification obtained. The resulted mixture was filtered then dried in air finally recrystallized using rectified spirit. Further carried out from purification was Ethyl Acetate & n-Hexane.
Chalcone derivatives (2a-k), malononitrile and anhydrous ammonium acetate were taken in RBF and absolute ethanol was used as solvent. This mixture was refluxed for 7-8 Hrs. then cooled to RT. As solution attained RT, solid was obtained. Filtration & washing was done with dist. H2O thoroughly, recrystallization from Ethanol to yielded compounds (3a-k)23.
Compound (3a-k) and excess of glacial CH3COOH were mixed and then refluxed condition for 7-8 Hrs. Glacial acetic acid was self-solvent. After the completion of reaction, solution was cooled to RT. Solid thus obtained, was filtered, then washed thoroughly with cold dist. H2O several times, dried, Dioxane was used for recrystallization to give compounds (4a-k).
Various derivatives of 3-(2-Chloroacetyl)-5-(2,4-substitutedphenyl)-2-Methyl-7-(2-Oxo-2H-Chromen-3-yl)Pyrido[2,3-d]Pyrimidin-4(3H)-one (5a-k) were synthesized from compounds (4a-j). Saturated solution of sodium acetate was prepared in glacial acetic acid. Compound (4a-k) was dissolved drop wise to the mixture and then was cooled at 0-5º C. Chloroacetyl chloride was dropwise added in the solution at 0-5º C during 1 Hr. time period. When addition was completed, this reaction mixture was heated at 80º C for 1.5 Hrs. & this solution was stirred at RT for 12 Hrs. which gave compounds (5a-k).
Various derivatives of N-(Hydroxyadamantan-1-yl)-5-(2,4-substituted phenyl)-2-Methyl-4-Oxo-7-(2-Oxo-2H-Chromen-3-yl)Pyrido[2,3-d]Pyrimidine-3(4H) carboxamide (6a-k) were synthesized by reaction between the mixture of 3-aminoadamantan-1-ol and K2CO3. This was refluxed for 5-6 hours, and then the solution was cooled to RT. Then this mixture was dumped into ice to obtain solid, which was filtered & washed with distilled water, dried and finally recrystallization was carried out using Ethanol to yield (6a-k) products.
The compounds were confirmed by study of FT-IR spectra, using KBr discs. on Perkin-Elmer 1600 FTIR, 1H NMR and 13C NMR spectra were measured on Bruker 500 MHz in DMSO -d6 as solvent was used and TMS – tetra methyl silane was internal standard respectively. LC-MS were carried out on LCMS. According to NMR data presence of methyl group showed value of δ near 2.92-3.09, proton of the secondary of NH group showed value of δ near 6.76-7.35, -OH group pf adamantane showed value of δ near 3.43-3.47, -OCH3 showed value of δ near 3.79-3.90 and 5H of coumarin showed value of δ 6.75-8.59. Figure 1 to 4 shows 1H NMR spectra of the compounds TT1, TT3, TT5 and TT8 respectively. Figure 5 shows 13C NMR spectra of the compound TT1. Figure 6 and 7 shows IR spectra of the compounds TT1 and TT3 respectively. Figure 8 represents LCMS spectra of the compounds TT1 and TT3 respectively.
Biological activity
Antibacterial activity
Table-3 shows MIC (minimum inhibition concentration) of the N-(3-Hydroxyadamantan-1-yl) – 5 – (2,4-substitutedphenyl)-2-Methyl-4-Oxo-7-(2-Oxo-2H-Chromen-3-yl)Pyrido[2,3-d]Pyrimidine-3(4H)Carboxamide (6a-k) (Graph-1). Majority of the molecules which were tested, showed noticeable activities against E. Coli, P. Aeruginosa, S. Aureus & S. Pyogenes. From the results of antibacterial study of these N-(Hydroxyadamantan-1-yl)-5-(2,4-substitutedphenyl)-2-Methyl-4-Oxo-7-(2-oxo-2H-Chromen-3-yl)Pyrido[2,3-d]Pyrimidine- 3(4H)carboxamide (6a-k) derivatives such as TT1 (R1 = -H and R2 = -OCH3), TT6 (R1 = -H and R2 = -Br), TT8 (R1 = -H and R2 = -CH3) and TT9 (R1 = -Cl and R2 = -Cl) showed better activity at 50µg/mL; TT3 (R1 = -H and R2 = (CH3)2N-) and TT10 (R1 = -Br and R2 = -H) showed better activity at 62.5µg/mL; TT2 (R1 = -H and R2 = -Cl), TT4 (R1 = -H and R2 = -OH), TT5 (R1 = -H and R2 = -NO2), TT7 (R1 = -OH and R2 = -H) and TT11 (R1 = -Br and R2 = -H) showed better activity at 100µg/mL against E. Coli as comparing with Ampicillin (MIC=100µg/mL).
N-(Hydroxyadamantan-1-yl)-5-(2,4-substitutedphenyl)-2-Methyl-4-Oxo-7-(2-Oxo-2H-Chromen -3-yl)Pyrido[2,3-d]Pyrimidine-3(4H) Carboxamide (6a-k) derivatives such as TT3 (R1 = -H and R2 = (CH3)2N-) showed better activity at 50µg/mL; TT1 (R1 = -H and R2 = -OCH3) and TT8 (R1 = -H and R2 = -CH3) showed better activity at 62.5µg/mL; TT2 (R1 = -H and R2 = -Cl), TT6 (R1 = -H and R2 = -Br), TT9 (R1 = -Cl and R2 = -Cl) and TT10 (R1 = -CH3 and R2 = -H) showed better activity at 100µg/mL against P. Aeruginosa as comparing with Ampicillin (MIC = 100 µg / mL) and equivalent as Chloramphenicol (MIC = 50 µg / mL).
N-(Hydroxyadamantan-1-yl)-5-(2,4-substitutedphenyl)-2-Methyl-4-Oxo-7-(2-Oxo-2H-Chromen -3-yl)Pyrido[2,3-d]Pyrimidine-3(4H) Carboxamide (6a-k) derivatives such as TT9 (R1 = -Cl and R2 = -Cl) showed better activity at 50µg/mL; TT1 (R1 = -H and R2 = -OCH3), TT4 (R1 = -H and R2 = -OH), TT10 (R1 = -Br and R2 = -H) and TT11 (R1 = -Br and R2 = -H) showed better activity at 100µg/mL against S. Aureus as comparing with Ampicillin (MIC = 100 µg / mL) and N-(3-Hydroxyadamantan-1-yl)-5-(2,4-substitutedphenyl)-2-Methyl-4-Oxo-7-(2-Oxo-2H-Chromen-3-yl)Pyrido[2,3-d]Pyrimidine-3(4H) Carboxamide (6a-k) derivatives such as TT1 (R1 = -H and R2 = -OCH3), TT2 (R1 = -H and R2 = -Cl) and TT8 (R1 = -H and R2 = -CH3) showed better activity at 62.5µg/mL; TT3 (R1 = -H and R2 = (CH3)2N-), TT9 (R1 = -Cl and R2 = -Cl) and TT10 (R1 = -Br and R2 = -H) showed better activity at 100µg/mL against S. pyogenes as compared to Ampicillin (MIC = 100 µg / mL).
Antifungal activity
The minimum inhibition concentration of the N-(3-Hydroxyadamantan-1-yl)-5-(2,4-substitutedphenyl)-2-Methyl-4-oxo-7-(2-Oxo-2H-Chromen-3-yl)Pyrido[2,3-d] Pyrimidine-3(4H) Carboxamide (6a-k) is shown in Table-4 (Graph-2). Most of the compounds tested, exhibited considerable activities against C. Albicans, A. Niger & A. Clavatus. Antifungal activity results of N-(3-Hydroxyadamantan-1-yl)-5-(2,4-substitutedphenyl)-2-Methyl-4-Oxo-7-(2-Oxo-2H-Chromen-3-yl)Pyrido[2,3-d]Pyrimidine-3(4H)-Carboxamide (6a-k) derivative such as TT2 (R1 = -H and R2 = -Cl) and TT6 (R1 = -H and R2 = -Br) showed better activity at 50 µg/mL; TT5 (R1 = -H and R2 = -NO2) and TT8 (R1 = -H and R2 = -CH3) showed better activity at 62.5µg/mL; TT1 (R1 = -H and R2 = -OCH3), TT4 (R1 = -H and R2 = -OH), TT7 (R1 = -OH and R2 = -H) and TT11 (R1 = -Br and R2 = -H) showed better activity at 100µg/mL against C. Albicans as compared Nystatin (MIC = 100 µg / mL) and Griseofulvin (MIC = 500 µg / mL). N-(3-Hydroxyadamantan-1-yl)-5-(2,4-substitutedphenyl)-2-Methyl-4-Oxo-7-(2-Oxo-2H-Chromen-3-yl)Pyrido[2,3-d]Pyrimidine-3-(4H)-Carboxamide (6a-k) derivative such as TT5 (R1 = -H and R2 = -NO2) better activity at 50 µg/mL; TT2 (R1 = -H and R2 = -Cl)showed better activity at 62.5µg/mL; TT1 (R1 = -H and R2 = -OCH3), TT3 (R1 = -H and R2 = (CH3)2N-), TT4 (R1 = -H and R2 = -OH), TT6 (R1 = -H and R2 = -Br), TT7 (R1 = -OH and R2 = -H) and TT11 (R1 = -Br and R2 = -H) showed better activity at 100µg/mL against A. Niger as comparing with Nystatin (MIC = 100 µg / mL) and Griseofulvin (MIC = 500 µg / mL). N-(3-Hydroxyadamantan-1-yl)-5-(2,4-substitutedphenyl)-2-Methyl-4-Oxo-7-(2-Oxo-2H-Chromen-3-yl)Pyrido[2,3-d]Pyrimidine-3(4H)-Carboxamide (6a-k) derivative such as TT1 (R1 = -H and R2 = -OCH3) and TT8 (R1 = -H and R2 = -CH3) showed better activity at 50µg/mL; TT5 (R1 = -H and R2 = -NO2) better activity at 62.5µg/mL; TT2 (R1 = -H and R2 = -Cl), TT6 (R1 = -H and R2 = -Br), TT10 (R1 = -Br and R2 = -H) and TT11 (R1 = -Br and R2 = -H) showed better activity at 100µg/mL against A. Clavatus as comparing with Nystatin (MIC = 100 µg / mL) and Griseofulvin (MIC = 500 µg / mL).
Anti Tubercular activity
Very promising results of antibacterial activity test of N-3-Hydroxyadamantan-1-yl)-5-(2,4-substitutedphenyl)-2-Methyl-4-Oxo-7-(2-Oxo-2H-Chromen-3-yl)Pyrido[2,3-d]Pyrimidine-3(4H)Carboxamide (6a-k) directed to study out more primary screening against M. tuberculosis. The antitubercular activity results of Pyrido[2,3-d]Pyrimidine derivatives (6a-k) presented in Table-4 (Graph-3). For the screening trials, concentration of exhibiting compounds was 1000, 500 and 250 µg/mL. From these, the compounds exhibiting good activity in the primary screening were considered for secondary screening against M. tuberculosis H37RV in the L. J. Medium. The results of the antitubercular activity were matched with Rifampicin at the concentration 40 µg/mL. N-(3-Hydroxyadamantan-1-yl)-5-(2,4-substitutedphenyl)-2-Methyl-4-Oxo-7-(2-Oxo-2H-Chromen-3-yl)Pyrido[2,3-d]Pyrimidine-3(4H) carboxamide (6a-k) such as TT1, TT7 and TT11 containing bromo, hydroxy and methoxy substituted derivatives exhibited M. tuberculosis MIC values in around 62.5 µg/mL producing 95-99% better results. But the other compounds exhibited moderate to poor activity against M. tuberculosis H37RV.
Anti Malarial activity
Antimalarial activity of Pyrido[2,3-d]Pyrimidine derivatives (6a-k) is shown in Table-5. Chloroquine and Quinine were the standard drugs used to compare antimalarial activity. The values of MIC are 0.020 µg/mL and 0.268 µg/mL respectively. Pyrido[2,3-d]Pyrimidine derivatives no. 1 & 2, 4 to 11 showed better activity at 0.88µg/mL, 0.25µg/mL, 0.98µg/mL, 0.74µg/mL, 0.42µg/mL, 0.35µg/mL, 0.46µg/mL, 0.31µg/mL, 0.52µg/mL and 0.23µg/mL respectivelyas antimalarial activity comparing with to Quinine (MIC=0.268 µg/mL).
Acknowledgement
Authors are thankful to Dr. A. S. Patel (Principal, Navyug science college, surat) who provided all facilities for research.
Conflict of Interest
There are no conflict of interest.
References
- D. J. Brown, The Chemistry of Heterocyclic Compounds, Weissberger, A., Ed.; The Pyrimidines, Wiley Intercedence, New York, 1970, Vol. 16.
CrossRef - D. T. Hurst, “An Introduction to the Chemistry and Biochemistry of Pyrimidines, Purines and Pteridines”, Wiley:Chichester, UK, 1980.
- J. T. Bojarski, J. L. Mokrosz, H. J. Barton, M. H. Paluchowska, Adv. Heterocycl. Chem., 1985, 38, 229.
CrossRef - E. A. Gunnewegh, A. J. Hoefnagel, R. S. Downing and H. van Bekkum, Recl. Trav. Chin. Pays Bas, 1996, 115, 226.
CrossRef - W. C. Sun, K. R. Gee and R. P. Haugland, Bio. Org, Med. Chem. Lett., 1998, 8, 3107.
CrossRef - J. Oyamadas, C. Jia, Y. Fujiwara and T. Kitamura, Med. Chem., Lett, 2002, 14, 380.
CrossRef - R. D. H. Murray, J. Medez and S. A. Brown, “The natural Coumarins, Occurrence, Chemistry and Biochemistry”, Wiley, New York, 1982, p.68.
- Gossnitzer, E., Feierl, G., Wagner, U., “Synthesis, structure investigations, and antimicrobial activity of selected s-tran-6-aril-4-isopropyl-2-2[2-[(E)-1 phenylalkylidene]-(E)-hydrazino]-1,4-dihy-dropyrimidine hydrochlorides”. Eur. J. P, 2002.
CrossRef - Hogale, M.B. et al., “Synthesis and biological activity of some urethane derivatives of chalcones”, Orient J. Chem. 1986, 2, 55–57.
- Mattew, J., Subba R. A. V., Rambhav S., “Propterol an antibacterial agent from Pterocarpus marsupium”, Curr. Sci. 1984, 53, 576–577.
- Ahluwalia, V.K. et al, “Synthesis and antimicrobial activity of substituted 3,4-dihydro-2H-1-benzopyrans”, Indian J. Chem. 1987, 26, 384–386.
CrossRef - Jani M. K., Shah B. R., Undavia N. K., Trivedi P. B., Chem. Abstr., 1994, 121, 35513p.
- Ishitsuka, H. et al., “Direct and specific inactivation of rhinovirus by chalcone Ro” 09-0410, Antimicrob. Agents Chemother. 1982, 22, 617– 621.
CrossRef - Ninomiya Y., Shimma N., Ishitsuka H., “Comparative studies on the ant rhino virus activity and the mode of action of the rhinovirus capsid binding agents, chalcone amides”, Antiviral. Res. 1990, 13, 61-74.
CrossRef - Calis, U., Koksal, M., “Synthesis and evaluation of anticonvulsant activities of some new arylhexahydropyrimidine-2,4-diones”, Arzneim.-Forsch./Drug Res. 2001, 51 (II), 523.
CrossRef - Alagarsamy, V., Solomon, V.R., Murugan, M., “Synthesis and pharmacological investigation of novel 4-benzyl-1-substituted-4H-[1,2,4]triazolo[4,3-a]quinazolin-5- ones as new class of H1-antihis-taminic agents”, Bio. org., Med. Chem., 2007, 15, 4009-40.
CrossRef - Crespo, M. I. et al., “Design, Synthesis, and Biological Activities of New Thieno[3,2-d]pyrimidines as Selective Type-4 Phosphodi-esterase Inhibitors”, J. Med. Chem., 1998, 41, 4021-4031.
CrossRef - Al Safarjalani, O. N., Zhou, X. J., Ras, R. H., Shi, J., Schinazi, R. F., Naguib, F. N. and El Kouni, M. H., Cancer Chemother. Pharmacol., 2005, 55, 541–551.
CrossRef - Magalhães T. F. F., da Silva C. M., dos Santos L. B. F., Santos D. A., Silva L. M., Fuchs B. B., Mylonakis E., Martins C. V. B., de Resende-Stoianoff M. A., de Fátima Â., “Cinnamyl Schiff bases: synthesis cytotoxic effects and antifungal activity of clinical interest”, Lett. Appl. Microbiol., 2020, 71 (5): 490-497.
CrossRef - Morsy N. M., Hassan A. S., Hafez T. S., Mahran M. R. H., Sadawe I. A., Gbaj A. M., “Synthesis antitumor activity enzyme assay DNA binding and molecular docking of Bis-Schiff bases of pyrazoles”, J. Iran. Chem. Soc., 2021, 18 (1): 47-59.https://doi.org/10.1007/s13738-020- 02004-y
CrossRef - T. B. Johnson, R. D. Coghill, J. Am. Chem. Soc., 1925, 47, 2838.
CrossRef - G. R. Wytt, Biochem. J., 1951, 48, 584.
CrossRef - G. R. Wytt, Biochem. J., 1951, 48, 584.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









