A Comparative Study of PANI and Its Copolymer Poly (aniline co o nitroaniline)
Yogesh Kumar Sharma* , Aruna Solanki
, Aruna Solanki , Himani Yadav
, Himani Yadav and Nitin Kumar
and Nitin Kumar
Department of Chemistry, UCOS, MLSU, Udaipur , Rajasthan, India.
Corresponding Author E-mail: nitinkumar@mlsu.ac.in>
DOI : http://dx.doi.org/10.13005/ojc/390228
Article Received on : 22 Mar 2023
Article Accepted on :
Article Published : 18 Apr 2023
Reviewed by: Dr. Baidaa K. Al-Rubaye
Second Review by: Dr. Wayan Sutapa
Final Approval by: Dr. Reyadh Ahmed
The investigation of chemical co-polymerization of aniline and ortho nitroanilinein acidic medium was done. Ammonium persulphate (APS) acted as the oxidizing agent. Synthesis of PANI and its copolymer was done followed by characterization using FTIR analysis to assess bonding pattern. Detection of copolymer, its crystallinity and orientation was achieved using XRD technique. To determine the heat resistance of the copolymer, TGA was employed. One of the prominent uses of synthetic poly (aniline-co-o-nitroaniline) is that it is used as a semiconducting material in many electronic devices.
KEYWORDS:Conductivity; Copolymerization; Thermal Stability; PANI
Download this article as:| Copy the following to cite this article: Sharma Y. K, Solanki A, Yadav H, Kumar N. A Comparative Study of PANI and Its Copolymer Poly (aniline co o nitroaniline). Orient J Chem 2023;39(2). |
| Copy the following to cite this URL: Sharma Y. K, Solanki A, Yadav H, Kumar N. A Comparative Study of PANI and Its Copolymer Poly (aniline co o nitroaniline). Orient J Chem 2023;39(2). Available from: https://bit.ly/3GNaEWb |
Introduction
Scientific advances have realized the importance of conductive polymers, which find use in a variety of domains like electronics, anti-corrosion coatings, antibacterial agents, absorbent materials etc. As a result, the synthesis and applications are being investigated regularly. Polymers under the category Aryl amines are one of the main synthetic macromolecules recognized by mankind 4. In particular, the availability and chemical stability of polyaniline and its derivatives have made them popular and have provided opportunities to researchers to optimize the properties in order to create new materials with improved desirable characteristics. In the past decade, several ways to modify PANI have been proposed, such as changing monomer units, copolymerization, changing doping agents, homopolymer evolution, and synthesizing polymer composites with various functional materials 6. Owing to its peculiar properties such as stability in the chemical environment, changeable electrical conductivity (by changing the pH during preparation), distinctive proton polarity, atypical redox activity and cost effectiveness; PANI has attracted the attention of the researches worldwide 7.
 |
Scheme 1: Polymerization of (a)aniline and (b) aniline 0-nitroaniline. |
In their purest forms polymeric conductors viz. poly thiophene, polyethylene and poly aniline (PANI) behave as semiconductors. However, upon being doped these exhibit high electrical conductivity 8. Besides, by means of co-polymerization the productivity of polyanilines, toluidine, anisidine etc can be increased. These properties are in turn, dependent on electron donating or electron withdrawing or alkyl groups present in the polymers. While the presence of electron withdrawing groups diminishes the electron density of the aniline ring, the electron donating groups tend to enhance the electron density of the phenyl ring. The electron density of the aromatic nucleus, however, remains less affected by the alkyl group. Some reports have suggested that the alkyl groups exhibit some tender positive inductive effect thereby increasing the electron density9. In the present work, synthesis of poly (aniline-co-ortho-nitro aniline) was pursued following its characterization using FTIR spectroscopy. Besides, emphasis was given on the effects caused by the presence of o-nitro aniline in the copolymer 10.
Materials and methods
Aniline (Merck) was double distilled. o-Nitroaniline (Cisco) and all reagents (AMS) and all the solvents used asmethanol, ethanol were of analytical grade. These were used after being purified.
Synthesis of polyanilne
The polymer synthesis was achieved using the following procedure: First of all 0.47 grams (0.1 moles) was made to dissolve in 1 molar solution of aniline hydrochloride. Subsequently, the organic solvent was poured in it along with continuous stirring. This mixture was left for five minutes after which 0.1 M ammonium sulphate (previously dissolved in 50 ml 1 M hydrochloric solution) was added. This initiated the polymerization of aniline. The resultant mixture obtained was subjected to stirring for eight hours at room temperature. Consequently, a precipitate was obtained which was filtered and collected. This precipitate was further subjected to repeated washings using distilled water and ethanol. The product so obtained was allowed to dry for 48 hours at room temperature under vacuum.
Synthesis of Coplymer Poly(aniline‑co‑o‑nitroaniline)
The copolymer was synthesized using the following procedure 10. Aniline solution (1M) was prepared by using 150 ml of hydrochloric acid having 2 M concentration. The solution of o-nitro aniline (3M) was prepared in 150ml hydrochloric acid (2M). After this, both the solutions were allowed to be in RB phase and magnetic flux was kept suitably. The monomer solution was cooled by adjusting the temperature to five degree centigrade. The oxidation process was initiated with the addition of the solution of APS whose molarity was 0.5M and was prepared in 2M hydrochloric acid. The beginning of the process of polymerization was marked by the appearance of a bright green color, which appeared after few minutes following addition of APS. The ratio of 1 mole to 3 moles (aniline: o-nitro aniline) was maintained so that copolymers of different compositions could be obtained. The reaction mixture was made to stand for 4 to 5 hours and subsequently for 20 hours at room temperature under an inert atmosphere. The ageing of this copolymerization reaction was done for further 24 hours so that the reaction could be completed. The dispersion and crushing of the copolymer precipitate was done very carefully.
Results and Discussion
FTIR Analysis of PANI and the synthesized Copolymer.
The FTIR spectrum obtained for the copolymer has been depicted in figure 1.A major band is visible in the region of 1595-1560 cm-1.This band corresponds to stretching vibration shown by the quinoid ring.One of the bands appearing near 1385 cm-1, which is broad in nature is because of the C-N vibration (of stretching nature) along the quinoid structure. In the quinoid-benzenoid-quinoid ring, there are C-N bonds in the alternating units and their stretching is represented by band at 1310 cm-1of average intensity. The fact that nature of C-C or C-N stretching is symmetrical is evident from the peak at 1210 cm-1. Moreover, the N-H bending mode is depicted by peak at 1304 cm-1. Major extension bands of the copolymer are asymmetric. The nitro group (NO2) in ortho nitro aniline has symmetric stretching and it correlates with peaks at 1510 and 1346 cm-1. Yet another peak at 1110 cm-1 is due to the in-plane bending of the C-H bond. This band provides a confirmation of presence of ortho nitro aniline moiety in the copolymer. Besides, it implies that there is a great deal of coupling between the nitro group and the ring. Owing to the hydrogen bonding between hydrogen atom of the amine group and oxygen atom of the nitro group, there is a compelling decrease in the intensity. The bands at 1145 and 831 cm-1 represent the in-plane and out-of-plane bending of C-H bond bands. Various bands along with their frequencies have been presented in Table 1.
Table 1: FTIR Peak of Copolymer
|
S.NO. |
Frequency |
Values |
|
1 |
Quinoid stretching |
1595 cm-1and 1560 cm-1 |
|
2 |
C-N Stretching |
1385 cm—1 |
|
3 |
C-N stretching(alternate) |
1310 cm—1 |
|
4 |
N-H Bending(w) |
1304cm-1 |
|
5 |
C-C Stretching |
1210 cm—1 |
|
6 |
Symmetric stretching |
1510 cm-1 |
|
7 |
Asymmetric stretching |
1346 cm-1 |
|
8 |
C-H Bending(in plane) |
1110 cm-1 |
|
9 |
C-H Bending(in plane) |
1145cm-1 |
|
10 |
C-H Bending(in plane) |
831cm-1 |
 |
Figure 1: FTIR spectra of PANI |
 |
Figure 2: FTIR spectra of Copolymer. |
TGA
The thermogravimetric analysis reveals that weight loss occurs by loss of water content and other impurities. The weight loss typically starts at 100oC. However, on moving from 100oC to 350oC, it is seen that the degradation is very less, thereby reflecting that the copolymer is thermally stable. The data further indicates that the Co-polymers from o-nitro aniline and aniline could be synthesized below 3500C. Furthermore, at a temperature above 400oC, the degradation of copolymer starts.
 |
Figure 3: TGA analysis of PANI. |
 |
Figure 4: TGA Analysis of Copolymer. |
XRD (X-ray Diffraction Technique)
The X-ray diffraction shows four diffraction peaks at 9270, 15160, 20650 and 25190. These belong to polyaniline at room temperature (figure 5).The diffraction pattern also shows a sharp peak due to the benzenoid and quinonoid groups in polyaniline. The XRD pattern is also suggestive of semi-crystalline nature of the polymer. Bragg’s Law and Debye Scherer equations were used to calculate inter planar crystalline distances and size of the crystallite. The crystallinity was obtained 36.15% and the size of crystal was found to be 2.10 nano meter.
 |
Figure 5: XRD spectra of THF 7ml |
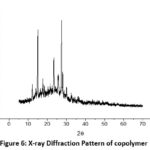 |
Figure 6: X-ray Diffraction Pattern of copolymer |
Conductivity of Copolymers
The conductivity of the copolymer is found to decrease as the concentration of aniline and nitro aniline in the mixture is increased. This is lower than the poly aniline (aniline-co-o-nitro aniline) as shown in Figures 7 and 8.
 |
Figure 7: Conductance of polyaniline |
 |
Figure 8: Conductance of Co-polymer |
Conclusion
The copolymerization of ortho nitro aniline took place with aniline by virtue of chemical oxidative polymerization with ammonium persulphate acting as oxidizing agent and medium being acidic in nature. The feed ratio was kept 1:3. Though polymerization of ortho nitro aniline was not uniform, high magnitude was not obtained for conductivity for copolyaniline-o-nitroaniline as compared to that of polyaniline.
Acknowledgement
The authors extend their humble acknowledgement towards the coordination Chemistry Lab, Department of Chemistry, MLS University, Udaipur, India. Special thanks are extended to RUSA.The studies involving characterization as TGA were done at MNIT, Jaipur and others involving Conductance, XRD and FTIR were done at UCOS, MLS University, Udaipur, India.
Conflict of Interest
It is declared by the authors that there is no conflict of interest.
References
- Deshmukh, V. B.; Paithankar, K. S.; Shelke U. N.; Gade V.K, Int. J. Sci. Res. in Physics and Applied Sciences.2018, 6(6),2348-3423
- Li, Z.; Li, J.; He, Y.; Zhou, K.; Ji, H.; Shi, W.; Sun, Y.; Wu, T.; Ge, D,Materials Science and Engineering.2019, 611,012037
CrossRef - Ramanavicius, S.; Ramanavicius, A.; Polymers.2021, 13, 49
CrossRef - Yana, Y.; Yangb, G.; Xu, J.L.; Zhangd, M.; Kuoe, C.C.; Wang, S.D, Science and Technology of Advanced Materials.2020, 21, 1, 768–786
CrossRef - Dalaeen, J.S.A.; Husain, A.; Shariq, M.U.; Ahmad, A, Indian Journal of Advances in Chemical Science.2021, 9(3), 174-180
- Ahmad, N.; Sultana, S.; Faisal, S.M.; Ahmed, A.; Sabira, S.; Khan, M.Z, RSC Adv. 2019, 9, 41135
CrossRef - Guo, N.; Cang, F.; Wang, Z.; Zhao, T.T.; Song, X.R.; Farris, S.; Li, Y.Y.; Fu, Y.J,Materials Science & Engineering.2021, 126,112143
CrossRef - Liu, C.; Chen, F.Y.; Tang, Y.B.; Huo, P.W, J Mater Sci: Mater Electron.2021, 32, 15211–15225
CrossRef - Babayan, V.; Kazantseva, N.E.; Moucka, R.; Stejskal, J, Cellulose.2017, 24, 3445–3451
CrossRef - Hu, S.C.; Zhou, Y.; Zhang, L..L.; Liu, S.J.; Cui, K.; Lu, Y.Y.; Li, K.N.; Li, X.D,J Mater Sci.2018, 53,301
CrossRef - Ganguly, S.; Bhawal, P.; Ravindren, R.; Das, N.C, Journal of Nanoscience and Nanotechnology.2018, 18, 7641–7669
CrossRef - Gill, N.; Gupta, V.; Tomar, M.; Sharma, A.L.; Pandey, O.P.; Singh, D.P, Composites Science and Technology.2020, 192,108113
CrossRef - Hassan, S.S.M.; Kamel, A.H.; Hassan, A.A.; Amr, A.E.; Naby, H.A.E.; Omar, M.A.A.; Sayed, A.Y.A,Molecules.2020, 25, 2721
CrossRef - Mousavi, S.M.; Hashemi, S.A.; Bahrani, S.; Yousefi, K.; Behbudi, G.; Babapoor, A.; Omidifar, N.; Lai, C.W.; Gholami, A.; Chiang, W.H,Int. J. Mol. Sci. 2021, 22, 6850
CrossRef - Seid, L.; Lakhdari, D.; Berkani, M.; Belgherbi, O.; Chouder, D.; Vasseghian, Y.; Lakhdari,N, Journal of Hazardous Materials.2022 423,126986
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









