Comparative Investigation of Thermodynamic parameters of Seafood Waste as an Inhibitor for Mild Steel corrosion in Varied Acidic Concentration
1Department of Chemistry, Velalar College of Engineering and Technology (Autonomous), Erode – 638 001, Tamilnadu, India.
2Department of Chemistry, Kandaswami Kandar’s College, Periyar University, Velur - 641 043, Tamilnadu, India.
Corresponding Author E-mail: phddatas15@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380527
Article Received on : 20 June 2022
Article Accepted on : 05 Sep 2022
Article Published : 16 Sep 2022
Reviewed by: Dr. Buhani
Second Review by: Dr. Ioana Stanciu
Final Approval by: Dr. MGH Zaidi
Mild steel (MS) is the material that is frequently used in various sectors because of its specific properties like ductility and malleability, however it corrodes readily when exposed to corrosive environments. Inhibition is the best method to prevent corrosion, since it may be adjusted or added in place without interrupting a process. The present study focused on the thermal behavior of Crab Shell extract (CSE) as corrosion inhibitor on the surface of the MS in 0.5M and 1M sulphuric acid medium. Temperature study was employed to test the inhibitory action of the extract on MS using weight loss measurement. The effectiveness of inhibition declines with the rise of temperature. In 0.5M H2SO4 at 303K, the maximum efficiency was observed. The nature of adsorption in both the acidic medium follows El-Awady adsorption isotherm. The activation and thermodynamic study revealed that the reaction is spontaneous in nature. The inhibition process is endothermic which is confirmed by the positive enthalpy values.
KEYWORDS:Adsorption; Corrosion; Crab shell; Mild steel; Temperature
Download this article as:| Copy the following to cite this article: Priya M, Senthil M, Selvi S. K, Ranjitha S. Comparative Investigation of Thermodynamic parameters of Seafood Waste as an Inhibitor for Mild Steel corrosion in Varied Acidic Concentration. Orient J Chem 2022;38(5). |
| Copy the following to cite this URL: Priya M, Senthil M, Selvi S. K, Ranjitha S. Comparative Investigation of Thermodynamic parameters of Seafood Waste as an Inhibitor for Mild Steel corrosion in Varied Acidic Concentration. Orient J Chem 2022;38(5). Available from: https://bit.ly/3DtlIXy |
Introduction
In modern industry, sulphuric acid is a commodity chemical and is used for the manufacturing of variety of products like automobile batteries, fertilizers, sulphonation agents etc., and also it is used as pickling agent to remove the surface impurities from metals and alloys. Mild steel is an in-demand material in the past few years due to its qualities such as ductility, weldability, malleability, machinability etc., 1,2 It is a cost-effective type of steel, which many industrialists prefer for their industrial projects. However, it is very sensitive towards acid, alkali and in salt solution. It will rust over time unless it is treated with some sort of protective coating to prevent corrosion. Synthetic inhibitors which is used initially for corrosion protection have some negative impact like toxicity and high cost.3 Researchers focused on green inhibitors because of its abundance, environmental safety and low cost. Sea food waste is the most abundant one which causes harmful impact on environment if it is not properly disposed. Crab shell consists of calcium carbonate, protein, chitin and carotenoids. In view of these points, the current research focuses on employing crab shell as an inhibitory source to protect mild steel from corrosion in two different sulphuric acid media. Based on the literature review, 0.5M and 1M concentration sulphuric acid is used for the investigation.
Experimental
Materials and methods
Preparation of test specimen and inhibitor
5x1cm2 size MS specimens were polished mechanically with emery paper followed by the process of degreasing and then washed with deionized water. The dried samples were stored in a desiccator and used for the experimental procedure.
Crab shells were collected from fish market. It is washed with plenty of water and shadow dried. The dried shells were crushed using a mortar. Refluxing 25gm of powdered crab shell for 3 hours with 500 ml of 0.5M H2SO4 and the solution was cooled overnight. It was then filtered and made up to 500ml to get 5% extract. From the stock solution, various inhibitor concentrations were prepared.
Weight loss measurements
Weight loss was measured at four different temperature (303K, 313K, 323K & 333K) after 3 hours of immersion period. Pre-weighed MS specimens were immersed in 100ml of 0.5M H2SO4 in absence and presence of inhibitor (0.1% to 0.5%) and placed in a thermostat for 3 hours at a specific temperature. After the elapsed time, the samples were removed, washed with deionized water and then with acetone. Accurate reweighing was done on the air-dried samples. Triplicate measurements were performed at all the temperatures.
The percentage of inhibition efficiency (ɳ) and surface area coverage (Ɵ) were calculated using the following formulae 1&24
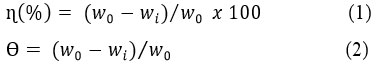
where, wo and wi is the weight loss values with and without inhibitor and Ɵ is the surface coverage of specimen by the inhibitor
Adsorption isotherms
Adsorption investigations provide insight into the character of adsorption of inhibitor on metal surfaces. Several adsorption isotherms can be employed to find out the type of interaction between metal surface and the inhibitor. In order to find out the best fit, El-Awady (modified Langmuir adsorption), Freundlich and Temkin adsorption isotherms were plotted based on the following expressions in linear forms given in 3,4 & 5 respectively.5
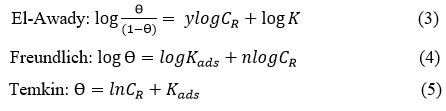
Kinetic and thermodynamic parameters
Energy of activation, entropy and enthalpy values were calculated using Arrhenius equation and Transition state equation 6 & 7
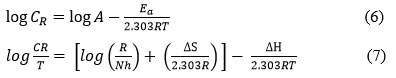
where, CR – corrosion rate, A – Arrhenius pre-exponential factor, Ea – activation energy, R – universal gas constant, T – temperature, N – Avogadro number, h – Planck’s constant, ΔS – entropy of activation and ΔH – enthalpy change.
From the graph of the transition state equation, the enthalpy change and the entropy change was evaluated from the slope (-ΔH/2.303R) and the intercept (log(R/Nh)+( ΔS/2.303R) respectively.6
The most important thermodynamic adsorption parameter ΔG was calculated by the following equation 8.
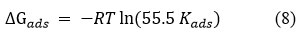
where, ΔG – free energy of adsorption, K – adsorption equilibrium constant, R – gas constant, T – absolute temperature and 55.5 – concentration of water in solution (molL-1)
Results and Discussion
Effect of Temperature
The efficiency of the inhibitory action of usually has a close relationship with its concentration and the environmental temperature.7 The graphical representation of the temperature study was given in Figure 1. Effect of temperature with various concentration of inhibitor in 0.5M and 1M acidic media are listed in Table 1. In both the concentration of acidic medium, the effectiveness of inhibition process decreases at subsequent higher temperatures, which means that there is an acceleration of desorption rate rather than adsorption at elevated temperature.8 The maximum efficiency was recorded with optimum concentration of 0.5% CSE at 303K in 0.5M sulphuric acid. Surface coverage is comparatively higher in 0.5M when compared to 1M solution.
Table 1: Influence of temperature on inhibition efficiency.
|
Concentration of acid |
Concentration of inhibitor (%) |
Inhibition efficiency (ɳ%) |
|||
|
303K |
313K |
323K |
333K |
||
|
0.5M H2SO4 |
Blank |
– |
– |
– |
– |
|
0.1 |
71.69 |
44.92 |
24.20 |
17.77 |
|
|
0.2 |
75.80 |
60.16 |
46.27 |
19.70 |
|
|
0.3 |
78.54 |
70.70 |
60.64 |
44.35 |
|
|
0.4 |
81.28 |
75.78 |
66.49 |
51.38 |
|
|
0.5 |
83.56 |
80.08 |
72.34 |
55.79 |
|
|
1M H2SO4 |
Blank |
– |
– |
– |
– |
|
0.1 |
60.37 |
21.44 |
20.78 |
10.14 |
|
|
0.2 |
69.31 |
45.58 |
40.03 |
26.60 |
|
|
0.3 |
75.78 |
63.41 |
57.35 |
36.76 |
|
|
0.4 |
79.13 |
68.88 |
62.81 |
49.69 |
|
|
0.5 |
81.37 |
74.68 |
69.87 |
53.87 |
|
 |
Figure 1: Influence of temperature on inhibition efficiency in (a) 0.5M and (b) 1M H2SO4. |
Adsorption isotherms
The nature of adsorption of inhibitor on MS surface can be predicted by the adsorption isotherms. In the present study, the three adsorption isotherms viz., El-Awady, Freundlich and Temkin adsorption isotherms were tried to find the best adsorption isotherm. Linear regression co-efficient values obtained from the plots of El-Awady, Freundlich and Temkin adsorption isotherms were found to be close to unity in both the concentrations. Comparatively higher R2 values found for El-Awady adsorption isotherm and the values were displayed in Table 2 which predicts the formation monolayer adsorption on MS surface9 and El-Awady isotherm was shown in Figure 2.
Table 2: R2 values of El-Awady adsorption isotherm.
|
Temperature (K) |
R2 Values |
|
|
0.5M H2SO4 |
1M H2SO4 |
|
|
303 |
0.9569 |
0.9909 |
|
313 |
0.9969 |
0.9875 |
|
323 |
0.9945 |
0.9919 |
|
333 |
0.9950 |
0.9904 |
 |
Figure 2: El-Awady adsorption isotherm for CSE in (a) 0.5M and (b) 1M H2SO4. |
Activation parameters
The Arrhenius and transition plot of inhibition process on MS in 0.5M and 1M H2SO4 is shown in Figure 3 and 4 respectively. Activation energy values, enthalpy and entropy values were determined and presented in Table 3. The activation energy values were higher in presence of CSE when compared with the absence of CSE for both the concentration leading to the conclusion that the inhibitor adsorbed on the surface of the MS was due to the increased energy barrier. Activation energy values less than 80KJ/mol suggests the process physical adsorption.10,11 Enthalpy reading that are positive predicts that the dissolution of metal is endothermic and the spontaneity of the adsorption process is indicated by the negative free energy values. The negative entropy values infers that the activated complex in the rate deciding step represents an association rather than detachment step, meaning that a diminish in disordering takes place on going from reactant to activated complex.12,13
Table 3: Kinetic and thermodynamic parameters of adsorption of CSE.
|
Conc. of acid |
Conc. of inhibitor (%) |
Ea (kJ/mol) |
-ΔG (KJ/mol) |
ΔH* (kJ/mol) |
-ΔS* (J/mol) |
||||||
|
303K |
313K |
323K |
333K |
||||||||
|
Blank |
33.11 |
– |
– |
– |
– |
13.23 |
192.65 |
||||
|
0.5M H2SO4 |
0.1 |
62.92 |
18.24 |
15.89 |
13.88 |
13.23 |
25.94 |
188.09 |
|||
|
0.2 |
66.60 |
17.03 |
15.69 |
14.16 |
11.66 |
27.61 |
187.53 |
||||
|
0.3 |
61.66 |
16.40 |
15.85 |
13.99 |
13.80 |
25.48 |
188.44 |
||||
|
0.4 |
62.23 |
16.10 |
15.78 |
13.85 |
13.78 |
25.74 |
188.41 |
||||
|
0.5 |
63.75 |
15.94 |
15.85 |
13.78 |
13.66 |
26.41 |
-188.21 |
||||
|
Blank |
42.58 |
– |
– |
– |
– |
39.96 |
190.41 |
||||
|
0.1 |
63.07 |
16.96 |
13.04 |
13.35 |
11.43 |
60.45 |
187.20 |
||||
|
0.2 |
65.14 |
16.20 |
14.16 |
14.00 |
12.74 |
62.53 |
186.98 |
||||
|
1M H2SO4 |
0.3 |
67.79 |
16.00 |
14.99 |
14.79 |
12.93 |
65.17 |
186.66 |
|||
|
0.4 |
66.01 |
15.76 |
14.88 |
14.63 |
13.60 |
63.39 |
187.03 |
||||
|
0.5 |
66.64 |
15.39 |
15.05 |
14.88 |
13.44 |
64.02 |
186.99 |
||||
 |
Figure 3: (a) Arrhenius and (b) Transition plot for CSE in 0.5M H2SO4. |
 |
Figure 4: (a) Arrhenius and (b) Transition plot for CSE in 1M H2SO4. |
Suggested Mechanism of inhibition
Organic compounds containing hetero atoms with π bonds could act as an efficient inhibitor against corrosion. Crab shell used for the present study, are rich in Calcium carbonate and protein. Calcium carbonate and other minerals was removed as residue during demineralization process. The protein content was hydrolysed as amino acid that consists of amino and carboxylic acid group and retain in the extract. The hetero atoms present in amino acid is responsible for the corrosion protection.14
Conclusion
Based on the results of the current work, the following conclusions were drawn:
CSE has a significant inhibitory effect on the surface of the MS in both 0.5M and 1M H2SO4. The efficiency of the inhibition declines with increase in temperature. The rate of adsorption is little bit higher in 0.5M H2SO4 when compared to 1M H2SO4. Linear regression co-efficient values of modified Langmuir adsorption isotherm were found to close to unity which supports the formation of monolayer on metal surface. Increased activation energy confirmed the process of adsorption of inhibitor in both the acidic medium. Positive enthalpy values and negative free energy values indicate the endothermic and spontaneous nature of the adsorption process, respectively, and inhibitors adsorb onto the metal surface through physical adsorption.
Acknowledgement
The authors are grateful to the institution for their support to do the research work.
Conflict of Interest
The authors affirm that there is no conflict of interest.
References
- S. Leelavathi, R. Rajalakshmi, J. Mater. Environ. Sci. 2013, 4(5), 625-638
- T. Laabaissi, H. Lgaz, H. Oudda, F. Benhiba, H. Zarrok, A. Zarrouk, A. El Midaoui, B. Lakhrissi, R. Touir, JMES, 2017, 8, 1054-1067
- O.O. Ogunleye, A.O. Arinkoola, O.A. Eletta, O.O.Agbede, Y.A.Osho, A.F.Morakinyo, J.O. Hamed, Heliyon, 2020, 6, e03205
CrossRef - Mayakrishnan Prabakaran, Seung-Hyun Kim, Venkatesan Hemapriya, Mayakrishnan Gopiraman, Ick Soo Kim, and Ill-Min Chung, RSC Advances, 2016, 6, 57144-57153
CrossRef - J.O. Madu, C. Ifeakachukwu, U. Okorodudu, F.V. Adams, I.V. Joseph, Journal of Physics: Conference Series, International Conference on Engineering for Sustainable World, 2019, 1378, 022092
CrossRef - A. Hamdy, Nour Sh. El-Gendy, Egyptian Journal of Petroleum, 2013, 22, 17-25
CrossRef - H. B. Ouici, O. Benali and A. Guendouzi,APMAS 2014, AIP conference proceedings, 2015, 1653, 020086
- A.S.Patel, V.A.Panchal, N.K.Shah, Bull. Mater. Sci., 2012, 35, 283-290
CrossRef - Xihua Xu, Ambrish Singh, Zhipeng Sun, K. R. Ansari and Yuanhua Lin, Royal society of chemistry, 2017, 4, 170933
CrossRef - Olatunde Alaba Akinbulumo, Oludare Johnson Odejobi, Ebenezer Leke Odekanle, Results in Materials, 2020, 5, 100074
CrossRef - I.E.Uwah, A.I.Ikeuba, B.U.Ugi, V.M.Udowo, Global Journal of Pure and Applied Sciences, 2013, 19, 23-31
CrossRef - R. Rajalakshmi, S. Subhashini, S. Leelavathi, Geethanjali, J. Nepal Chem. Soc., 2010, 25, 29-36
CrossRef - S. S. Shivakumar and K. N.Mohana, International Journal of Corrosion, 2013, 1-13
CrossRef - S.Subhashini, R.Rajalakshmi and A.S.Safina, Mat. Sci. Res. India., 2008, 5(2), 375-382

This work is licensed under a Creative Commons Attribution 4.0 International License.










