Biogenic Synthesis of Copper Oxide and Zinc Oxide Nanoparticles Using Catharanthus roseus L. Flower Extract and Evaluation of its Antioxidant and Antibacterial Properties
M. Kavitha1 , K. Shenbagam2
, K. Shenbagam2 , R. Kanmani1
, R. Kanmani1 and S. Philomina Mary1
and S. Philomina Mary1
1Department of chemistry, Holy Cross College (Autonomous), Affiliated to Bharathidasan University, Tiruchirapalli-620002, Tamil Nadu-India.
2Department of chemistry, Cauvery College for Women (Autonomous), Affiliated to Bharathidasan University, Tiruchirapalli-620018, Tamil Nadu-India.
Corresponding Author E-mail: kavitha2018@hcctrichy.ac.in
DOI : http://dx.doi.org/10.13005/ojc/380533
Article Received on : 20 Jul 2022
Article Accepted on : 27 Oct 2022
Article Published : 01 Nov 2022
Reviewed by: Dr. Selom Evenamede
Second Review by: Dr. Mariya Saani
Final Approval by: Dr. Tawkir Sheikh
Inorganic nano-metal oxides may be efficient replacements for organic antibiotics that are drug-resistant due to their wide range antibacterial activity towards pathogenic and mutagenic bacterial species. In this work, zinc and copper oxide nanoparticles were produced using Catharanthus roseus flower extract. It is a feasible alternative to other techniques because the biosynthesized nanomaterials made from plant extract are non-toxic and harmless to the environment. The X-ray diffraction (XRD), scanning electron microscopy (SEM), UV-Vis, and Fourier transform infrared spectrometry (FT-IR) investigations were achieved to confirm CuO and ZnO nanoparticles produced. In the DPPH experiment, the antioxidant properties of CuO and ZnO nanoparticles performed well. Gram-positive and gram-negative microorganisms are both effectively combatted by the produced copper and zinc oxide nanoparticles. The findings suggest that CuO nanoparticles were more effective towards microorganisms than ZnO nanoparticles.
KEYWORDS:Antibacterial activity; Assay; Catharanthus roseus; DPPH; EDAX; FTIR; SEM; UV-Vis; XRD
Download this article as:| Copy the following to cite this article: Kavitha M, Shenbagam K, Kanmani R, Mary S. P. Biogenic Synthesis of Copper Oxide and Zinc Oxide Nanoparticles Using Catharanthus roseus L. Flower Extract and Evaluation of its Antioxidant and Antibacterial Properties. Orient J Chem 2022;38(5). |
| Copy the following to cite this URL: Kavitha M, Shenbagam K, Kanmani R, Mary S. P. Biogenic Synthesis of Copper Oxide and Zinc Oxide Nanoparticles Using Catharanthus roseus L. Flower Extract and Evaluation of its Antioxidant and Antibacterial Properties. Orient J Chem 2022;38(5). Available from: https://bit.ly/3sIFXtT |
Introduction
A continuing quest for novel replacements has resulted from the proliferation of drug-resistant pathogens1. Among these infections, bacterial pathogens in water pose a serious concern to healthcare system since they are accountable for illnesses like diarrhoea, which account for 2195 new born global deaths worldwide and becomes bigger every day2. It has been shown that nanostructures are toxic to a variety of bacterial pathogens that produce disease3. Inorganic nanoparticles have a well-known widespread bactericidal action, although the mechanism behind this effect is still unclear5. It has recently been suggested that reactive oxygen species produced by the discharge of ions in solution are hazardous to microorganisms. Several investigations have discovered that because of its small size, nanoparticles may enter bacterial cell walls and damage organelles, which results in cell death. In contrast to their organic cousins, inorganic antibiotics may target several infections to battle their resistance via mutation6. Metal oxide nanoparticles belong to the inorganic nanoparticles that are most often utilized due to its antibacterial properties7. This is frequently owed to the statistic that metal oxides are more affordable than metal nanoparticles like silver and gold have a straightforward synthesis route that can be manipulated to modify the size and form of the nanoparticles8. Copper and zinc oxides are appropriate substitutes for antimicrobials obtained from organic materials. Numerous elements, including size, shape, and other elements mostly determined by synthetic means, affect their antibacterial effects9. Because of their small size and strong reactivity, metal nanoparticles may quickly infiltrate through bacterial cell walls and connect to proteins and interior organelles, which causes bacterial death10,11.
When tested against various bacterial species, copper oxide and zinc oxide had distinct antibacterial activities12. Because of their antibiotic properties, they remained employed in packaged foods, surface coatings, and wound healing13,14. CuO nanoparticles have shown therapeutic properties like antioxidant, immunomodulatory, sunscreen, and anticancer effects in addition to the antibacterial activities of these nanomaterials. The effectiveness of copper oxide nanoparticles in the medical area has been demonstrated15. This study examined the antibacterial efficacy of copper and zinc oxide nanoparticles towards both gram-positive and gram-negative bacteria.
The present study has shown that copper oxide, zinc oxide, and a plant extract have therapeutic promise, especially in terms of their antibacterial property. The Catharanthus roseus flower is used to treat eye issues and has been shown to have anti-tumor and therapeutic properties. Alstonine, a component of the root, is used to lower blood pressure. The antibacterial efficacy of copper and zinc oxide nanoparticles towards cultures of Staphylococcus aureus and pseudomonas aeruginosa was investigated in vitro. Zinc oxide nanoparticles are found in sunscreen. It has been shown that ZnO nanoparticles have antibacterial effects towards common foodborne illnesses16-18.
Experimental Methods
Catharanthus roseus fresh flowers were gathered from the local area of Tiruchirappalli District, cleaned with deionized water, and boiled with distilled water19.
Synthesis of Copper oxide nanoparticles
CuCl2.2H2O subsequently dissolved in 10 mL of floral extract solution for this plant component, and the combination was let to settle for three hours at room temperature. The reaction mixture was then combined with 1mL of a 10% NaOH solution. The substance that had precipitated was filtered and dried. In the oven, the raw product was reserved at 150°C for 12 hrs. The powder was obtained and calcined at 450°C for six hours20.
Synthesis of Zinc oxide nanoparticles
Anhydrous ZnCl2 (0.1g) was dissolved in deionized water and combined with a 10 mL Catharanthus roseus floral extracts for 3 hours at room temperature to create zinc oxide nanoparticles. The mixture was then poured in 1mL of a 10 % NaOH solution, and it was filtered and dried thereafter. The raw product spent 12 hours in the oven at 150oC. The obtained product was calcined for six hours at 450°C.
Characterization of Copper and Zinc oxide nanoparticles
Employing UV-visible spectroscopy in the 200 to 800 nm range, the copper oxide and zinc oxide nanoparticles were examined. The crystalline structure of the CuO and ZnO Nanoparticles was determined via X-ray diffraction spectroscopic (XRD) analysis. The elements contained in nanoparticles were recognized using energy dispersive X-ray spectroscopy (EDAX) coupled with FE-SEM. Scanning electron microscopy (SEM) was used to examine the nanoparticles’ structural details. The Fourier transform infrared spectra of the nanoparticles were documented on an FT-IR spectrometer ranges over 400 and 4000 cm-1,.
Antioxidant activity by DPPH method
Activity that scavenges free radical for the synthesized nanoparticles were tested by DPPH method. The samples were prepared in 20, 40, 60 and 80 µg/ml concentration and combined with DPPH for 30 minutes of incubation in dark and absorbance was recorded at 517 nm.
% of antiradical activity = (A-B) / A x 100
Where,
A = control absorbance; B = Sample absorbance
Antibacterial activity determination
The antimicrobial property of CuO and ZnO nanoparticle were analyzed through disc diffusion method against Staphylococcus aureus B23, and Pseudomonas aeruginosa 424.The inhibition zone was determined for microorganism that are gram positive and gram negative and compared with that of standard chloramphenicol. Whatmann No.1 sterile channel paper plates (6 mm width) were impregnated with required grouping of fluid and ethanolic extricates and put on the immunized agar. All of the plates underwent 24-hour hatching at 37 °C. Restraint zones were estimated and contrasted with the standard. Assessment of antibacterial movement was estimated through the breadth of the zones of restraint against the tried strains of microbes.
Results and discussion
The flower of Catharanthus roseus was collected near Trichy and described using the Flora of the Madras presidency, and the fresh flower extract exhibits significant role in the synthesized of CuO and ZnO nanoparticles.
The fluorescence analysis of the Catharanthus roseus flower powder.
In day light and ultraviolet light, the fluorescence activity of drug powder with the following chemicals was observed, which was found to offer different shades of color. The presence of alkaloids and flavones is indicated by the brown and red while the presence of sterols is indicated by the green fluorescence.
Table 1: The fluorescence analysis of the Catharanthus roseus flower powder.
|
S.NO |
Treatment |
Catharanthus roseus |
|||
|
24 Hours |
48 Hours |
||||
|
UV Light |
Day Light |
UV Light |
Day Light |
||
|
1 |
Powdered drug |
Green |
Green |
Green |
Green |
|
2 |
Powdered drug + Hexane |
Green |
Pale Green |
Pale Green |
Light Yellow |
|
3 |
Powdered drug + Benzene |
Pale Green |
Pale Green |
Pale Green |
Pale Green |
|
4 |
Powdered drug + Chloroform |
Pale Green |
Pale Green |
Pale Green |
Pale Green |
|
5 |
Powdered drug + Ethyl acetate |
Pale Green |
Pale Green |
Pale Green |
Pale Green |
|
6 |
Powdered drug + Alcohol |
Pale Green |
Pale Green |
Pale Green |
Pale Green |
|
7 |
Powdered drug + Acetone |
Green |
Pale Green |
Green |
Green |
|
8 |
Powdered drug + 50% H2SO4 |
Black |
Dark Brown |
Black |
Dark Brown |
|
9 |
Powdered drug + 1 N HCl |
Pale Green |
Light Brown |
White |
Light Brown |
|
10 |
Powdered drug + Aq. 1N NaOH |
Red |
Dark Red |
Green |
Red |
|
11 |
Powdered drug + Alc. 1N NaOH |
Green |
Pale Green |
Green |
Pale Green |
|
12 |
Powdered drug + H2O |
Red |
Red |
Green |
Dark Orange |
Table 2: Preliminary phytochemical screening of drug powder and various extracts of Catharanthus roseus.
|
S.No |
Phyto |
Results |
|||||
|
Plant Powder |
Hexane Extract |
Chloroform Extract |
Methanollic |
Ethanolic Extract |
aqueous Extract |
||
|
1 |
Alkaloid |
+ |
+ |
+ |
+ |
+ |
– |
|
2 |
Tannin |
+ |
– |
– |
+ |
+ |
+ |
|
3 |
Quinones |
+ |
+ |
– |
– |
– |
– |
|
4 |
Flavones |
+ |
– |
– |
+ |
+ |
+ |
|
5 |
Terpene |
+ |
+ |
+ |
+ |
+ |
– |
|
6 |
Coumarin |
– |
– |
+ |
– |
+ |
+ |
|
7 |
Sterol |
+ |
+ |
+ |
+ |
+ |
– |
|
8 |
Lignin |
+ |
+ |
+ |
+ |
– |
– |
|
9 |
Saponin |
+ |
– |
+ |
+ |
+ |
– |
|
10 |
Carbohydrate |
+ |
+ |
+ |
+ |
+ |
+ |
|
11 |
Phenol |
+ |
+ |
+ |
+ |
+ |
+ |
Quantitative analysis of phytochemicals
From the table major secondary metabolites such as phenol, flavonoids and terpenes are present. Compare to other two, plant have terpenes in high amount (Table 3).
Table 3: Major metabolites- Quantitative analysis.
|
S. No |
Secondary Metabolites |
Amount (mg/g) |
|
1 |
Phenol |
1.24 |
|
2 |
Flavanoids |
2.40 |
|
3 |
Terpenes in plant powder |
31.66 |
|
4 |
Terpenes in Hexane extract |
70.00 |
Optical characterization
Color variation in the mixture was used to visually track the reduction of Cu2+ ions to CuO Nanoparticles by Catharanthus roseus flower extract. It was found that the color of the solution gradually transformed from pale green to sky blue. Similarly, Zn2+ ions is reduced to ZnO Nanoparticles. The steady color shift in the reaction mixture from light green to pale yellow.
UV-Visible spectroscopy
In CuO Nanoparticles showed absorption peak at 390nm specifies the distinction Surface Plasma Resonance band for copper oxide nanoparticles size was less. In ZnO nanoparticles absorption peak was found at 374 nm leads the individual SPR bands for ZnO nanoparticles with less size.
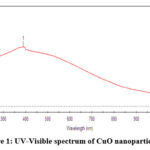 |
Figure 1: UV-Visible spectrum of CuO nanoparticle. |
 |
Figure 2: UV-Visible spectrum of ZnO nanoparticle |
FT-IR spectroscopy
Different functional groups were present, according to FT-IR analyses. It was evident that ZnO vibrations were present in the band at 422.08 cm-1. The vibrational band for CuO at 428.83 cm-1.
 |
Figure 3: FT-IR spectrum of CuO nanoparticle |
 |
Figure 4: FT-IR spectrum of ZnO nanoparticle |
SEM and EDAX characterization of metal
The ZnO and CuO nanoparticles are synthesized by using Catharanthus roseus 5µm and 3µm in size and both were spherical shape.
 |
Figure 5: Scanning Electron Microscope picture of ZnO Nanoparticles |
 |
Figure 6: Scanning Electron Microscope picture of CuO Nanoparticles. |
EDAX analysis verified the elemental composition of the produced CuO and ZnO Nanoparticles. CuO Nanoparticles were produced as the appearance of the copper and oxygen peaks in the EDAX spectrum, while ZnO Nanoparticles were produced as the zinc and oxygen peak.
 |
Figure 7: EDAX – CuO Nanoparticles. |
 |
Figure 8: EDAX – ZnO Nanoparticles |
XRD analysis
Figure 9 displays the XRD image of CuO Nanoparticles produced from Catharanthus roseus flower extract. The CuO Nanoparticles’ monoclinic structure was revealed by the diffraction peaks 2ɵ=33.53o, 35.82o, 38.78o, 48.99o, 55.75o, 58.64o, 62.26o and 67.34o, which were correspondingly indexed to the (110), (111), (111), (112), (020), (113), (311), and (220) planes. The difraction peaks that were obtained corresponded to the JCPDS (048-1548) of typical CuO NanoParticles.
 |
Figure 9: XRD spectrum of CuO Nanoparticles |
Figure. 10 illustrates the XRD pattern of Catharanthus roseus flower extract made from ZnO Nanoparticles. The difraction 2θ=32.630, 34.720, 36.480, 45.360, 53.750, 56.840, 63.260, and 66.460 was indexed to the monoclinic planes of ZnO Nanoparticles in the (100), (002), (101), (104), (102), (110), (103), (200), (112), (201), (004), and (202), respectively. The diffraction peaks that were obtained matched JCPDS21.
 |
Figure 10: XRD spectrum of ZnO Nanoparticles |
Antioxidant activity
In-vitro antioxidant property of synthesized CuO and ZnO Nanoparticles from catharanthus roseus flower extract by DPPH method is shown in Table 4. Ascorbic acid was utilized as a standard. The antioxidant activity at various concentrations say, 20, 40, 60 and 80 µg/ml are shown. The percentage inhibition(%) for standard ascorbic acid at 80 µg/ml is 98.23 whereas for CuO nanoparticles is 77.27 and for ZnO nanoparticles is 78.1. The results clearly indicates that copper oxide and zinc oxide nanoparticles synthesized using Catharanthus roseus have high capacity in controlling the free radicals.
Table 4: Antioxidant Activity of metal oxide nanoparticles by DPPH method
|
Concentration, µg/ml |
CuO Nanoparticles |
ZnO Nanoparticles |
Ascorbic acid |
|
20 |
22.72±0.59 |
21.87±0.53 |
41.0±0.90 |
|
40 |
40.90±0.86 |
43.75±0.06 |
68.10±0.60 |
|
60 |
63.63±0.45 |
56.25±0.93 |
84.64±0.80 |
|
80 |
77.27±0.40 |
78.12±0.45 |
98.23±0.30 |
Values are expressed as Mean ± SE (n=3)
Antibacterial activity
Copper and zinc oxide nanoparticles’ antibacterial potential was evaluated. Disk diffusion method was utilized to test the antibacterial assessment of copper and zinc oxide nanoparticles towards Staphylococcus aureus B23 and Pseudomonas aeruginosa 424. Both gram positive and gram negative bacterial organisms had their zones of inhibition established, and they were compared to those of conventional chloramphenicol. The synthesized CuO nanoparticle showed significant activity (zone of inhibition 12.83mm at 100μg/ml) compared with standard (zone of inhibition 15.00mm) for gram positive pathogen. The synthesized ZnO nanoparticle showed significant activity (zone of inhibition 11.33mm at 100μg/ml) compared with standard (zone of inhibition 15.83mm) for gram positive pathogen. CuO nanoparticle showed significant activity (zone of inhibition 10.27mm at 100μg/ml) compared with standard (zone of inhibition 15.93mm) for gram positive pathogen. ZnO nanoparticle showed significant activity (zone of inhibition 10.43mm at 100μg/ml) compared with standard (zone of inhibition 14.67mm) for gram negative pathogen. Both of these two have remarkable antimicrobial activity than ethanolic flower extract of Catharanthus roseus in both bacterial microbes. The antibacterial action of synthesized metal oxide nanoparticles was shown in the Table 5,6,7 and 8.
Table 5: Antibacterial potential of copper oxide nanoparticles towards Staphylococcus aureus
|
GPB |
Nanoparticles |
Zone of inhibition |
Ethanol Extract/ Control |
Zone of inhibition |
|
Staphylococcus aureus B23 |
Positive Control |
15.00±0.00 |
Positive Control |
16.00±0.29 |
|
Negative Control |
0.12±0.06 |
Negative Control |
1.11±0.02 |
|
|
CuONp /25 |
6.53±0.32 |
EECO /25 |
4.60±0.31 |
|
|
CuONp /50 |
8.23±0.15 |
EECO /50 |
6.93±0.23 |
|
|
CuONp /75 |
8.50±0.29 |
EECO/75 |
7.17±0.44 |
|
|
CuONp /100 |
12.83±0.44 |
EECO/100 |
9.13±0.24 |
Values are expressed as Mean ± SE (n=3)
Table 6: Antibacterial potential of zinc oxide nanoparticles towards Staphylococcus aureus
|
GPB |
Nanoparticles |
Zone of inhibition |
Ethanol Extract/ Control |
Zone of inhibition |
|
Staphylococcus aureus B23 |
Positive Control |
15.83±0.17 |
Positive Control |
16.43±0.30 |
|
Negative Control |
0.11±0.02 |
Negative Control |
1.38±0.01 |
|
|
ZnONp /25 |
2.83±0.17 |
EECO /25 |
1.67±0.35 |
|
|
ZnONp /50 |
6.17±0.17 |
EECO /50 |
2.07±0.12 |
|
|
ZnONp/75 |
9.17±0.60 |
EECO/75 |
6.17±0.66 |
|
|
ZnONp/100 |
11.33±0.33 |
EECO/100 |
9.07±0.58 |
Table 7: Antibacterial potential of copper oxide nanoparticles towards Pseudomonas aeruginosa.
|
GNB |
Treatment/Control |
Zone of inhibition |
Treatment/ Control |
Zone of inhibition |
|
Pseudomonas aeruginosa, 424 |
Positive Control |
15.93±0.23 |
Positive Control |
15.93±0.23 |
|
Negative Control |
0.21±0.03 |
Negative Control |
1.48±0.03 |
|
|
CuONp /25 |
3.23±0.15 |
EECO /25 |
2.23±0.15 |
|
|
CuONp /50 |
6.40±0.26 |
EECO /50 |
4.40±0.26 |
|
|
CuONp /75 |
8.67±0.18 |
EECO/75 |
6.67±0.18 |
|
|
CuONp /100 |
10.27±0.18 |
EECO/100 |
8.27±0.18 |
Values are expressed as Mean ± SE (n=3)
Table 8: Antibacterial potential of zinc oxide nanoparticles towards Pseudomonas aeruginosa
|
GNB |
Treatment/Control |
Zone of inhibition |
Treatment/Control |
Zone of inhibition |
|
Pseudomonas aeruginosa, 424 |
Positive Control |
14.67±0.17 |
Positive Control |
16.13±0.09 |
|
Negative Control |
0.11±0.04 |
Negative Control |
1.52±0.01 |
|
|
ZnONp /25 |
0.57±0.28 |
EECO /25 |
1.67±0.12 |
|
|
ZnONp /50 |
6.07±0.07 |
EECO /50 |
3.60±0.12 |
|
|
ZnONp/75 |
8.73±0.27 |
EECO/75 |
7.27±0.18 |
|
|
ZnONp/100 |
10.43±0.30 |
EECO/100 |
9.40±0.06 |
 |
Figure 11: Antibacterial activity of Copper oxide and Zinc oxide nanoparticles. |
Conclusion
The copper and zinc oxide nanoparticles were produced through fresh flower extract of Catharanthus roseus. The synthesized nanoparticles were confirmed by using various spectrometric techniques. The antioxidant study by DPPH method showed good result compared with standard ascorbic acid. The in-vitro antibacterial activity depicts the effective antibiotic action of both of these metal oxide nanoparticles. It concludes that CuO and ZnO nanoparticles helps for medication development.
Acknowledgement
The authors thank the Biotech Research Center and Holy Cross College (Autonomous), Trichy for permitting us to do this research work in their laboratory.
Conflict of Interest
All the authors declare that there is no conflict of interest.
References
- Pandey, P.; Packiyaraj, M.S.; Nigam. H.; Agarwal G.S.; Singh, B.; Patra, M. K. Beilstein J. Nanotechnol. 2014, 5, 789–800
- Kanmani , R.; Aarthi, M. Int. of Sci. and Engg. Res., 2016, 7(8), 131-137
- Kar, S.; Bagchi, B.; Kundu, B.; Bhandary, S.; Basu, R.; Nandy, P.; Das, S. Biochimica et Biophysica Acta. (2014), 1840, 3264–3276
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M. Molecules. 2015,20(5), 8856-8874
- Foldbjerg, R.; Jiang, X.; Miclăuş, T.; Chen, C.; Autrup, H., Beer, C. Toxicol. Res. 2015, 4(3), 563–575
- Bondarenko ,O.; Juganson, K.; Ivask, A.; Kasemets,K.; Mortimer, M.; Kahru, A. Arch Toxicol. 2013, 87(7), 1181-200
- Kumar, A.; Saxena, A.; De, A.; Shankara, R.; Mozumdar, S. RSC. Adv. 2013, 3(15) 5015-5021
- Ahmed, S.; Ahmad, M.; Swami, B. L.; Ikram, S. J. Adv. Res. 2016, 7(1), 17-28
- Sahooli, M.; Sabbaghi, S.; Saboori, R. Mater. Lett. 2012, 81, 169–172.
- Palza , H. Int. J. Mol. Sci. 2015, 16, 2099-2116;
- Agnihotri, S.; Mukherji, S.; Mukherji S. RSC Adv. 2014, 4, 3974–3983
- Zhang, J. International Coriference on Electronics and Optoelectronics, (ICEOE 2011), 2011, V3-94–V3-98, 94–98
- Topnani, N.; Kushwaha, S.; Athar, T. Int. J. Green Nanotechnol. Mater. Sci. Eng. 2010, 1(2), M67- M73
- Konieczny, J.; Rdzawski, Z. Arch.Mater. Sci. Eng. 2012, 56(7), 53–60.
- Bharathy M. S.; Jeyaleela, G. D.; Vimala, J. R.; Agila, A.; Hemadevi, M. Qriental J. of Chem. 2021, 37(4), 991-996.
- Prasad, T. N. V. K. V. .; Elumalai, E. K.; Khateeja, S. Asian Pac. J. Trop. Biomed. 2011,82-85.
- Kotakadi, V. S.; Raoa, Y. S.; Gaddamb, S. A.; Prasadc, T.N.V.K.V.; Reddy, A. V.; Sai Gopal, D.V.R.; Colloids Surf. B: Biointerfaces. 2013, 105, 194–198
- Raveendran, P.; Fu, J.; Wallen, S. L. J. Am. Chem. Soc. 2003, 15, 13940-13941
- Naika, H. R.; Lingaraju. K.; Manjunath, K.; Kumar G. D.; Nagaraju, G.; Suresh, D.; Nagabhushana,H. J. Taibah Univ. Sci. 2015, 9, 7-12
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Mater. Lett. 2012, 71, 114-116
- Dahrul, M.; Alatas, H.; Irzaman, Procedia Environ. Sci. 2016, 33, 661–667

This work is licensed under a Creative Commons Attribution 4.0 International License.









