Screening of Newspaper Pulp as a Potential Adsorbent for Impounding Pb2+ Ion from Aqueous Hinterlands
Department of Chemistry, PSGR Krishnammal College for Women, Peelamedu, Coimbatore, Tamil Nadu, India.
Corresponding Author E-mail: shyamalapsgr@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380410
Article Received on : 27 May 2022
Article Accepted on :
Article Published : 06 Jul 2022
Reviewed by: Dr. Iqbal Khalaf
Second Review by: Dr. Sandeep Vardhan
Final Approval by: Dr. Yashas S R
Intensification of industrial activity, environment stress contributes to the significant rise of heavy metal pollution in water resources. Pollution due to malignant heavy metals such as lead, chromium, arsenic etc., has been tremendously focused on communal health. Lead pollution-potable water finds great threat by different sources like plating units, lead pipes etc., Newspaper is a cellulosic materials, containing cellulose, hemicellulose and other inorganic fillers. The present study evaluates the potentiality of modified newspaper pulp for sequestration of Pb(II) ions. Characterizations of loaded and unloaded pulp were evaluated by FTIR, SEM and EDAX assay. Batch experimental studies were accomplished to assess the equilibration between the sorbate-sorbent through various operating factors viz., pH, dosage, time course, initial concentration, influence of ions, co-ions and effect of temperature. The residual concentrations of the Pb2+ ions from aqueous solutions are examined by UV-Visible Spectrophotometer. The equilibrium data was appropriately fitted with Langmuir adsorption isotherm model.
KEYWORDS:Batch Studies; Isotherms; Lead; Newspaper; Pulp
Download this article as:| Copy the following to cite this article: Devi N. S, Sri C. S. Screening of Newspaper Pulp as a Potential Adsorbent for Impounding Pb2+ Ion from Aqueous Hinterlands. Orient J Chem 2022;38(4). |
| Copy the following to cite this URL: Devi N. S, Sri C. S. Screening of Newspaper Pulp as a Potential Adsorbent for Impounding Pb2+ Ion from Aqueous Hinterlands. Orient J Chem 2022;38(4). Available from: https://bit.ly/3nNIznL |
Introduction
In accordance with environment, water is the most essential source for well-being of living organisms. As a result of distinct effects on the water resources, especially contaminants through human activities such as heavy metals, sewage discharges, disease causing agent, organic and inorganic pollutants, water is being polluted and still it is used unconsciously. The major sources of heavy metals from electroplating industries, tanneries, mining, textile, battery 1. Wide methods of treatment have been employed for curbing of heavy metals from waste water. The methods include coagulation, chemical precipitation, filtration, membrane separation, adsorption, ion exchange. Above all the techniques, adsorption is a promising and possible technique for heavy metal removal comprising high efficiency with a low cost approach2.The current study is aimed at the assessment screening of a low cost biomaterial for a potential sorption towards heavy metal ions. Large number of low cost eco-materials viz., sawdust, coconut husk, rice husk etc.,3, 4 Newspaper pulp, a source of cellulose, is chosen as an adsorbent material. The utilization of newspaper pulp for curbing Pb(II) ions from wastewater is considered to be an exclusive study.
Lead is a malignant pollutant in air and in drinking water. The presence of high quantity of lead in plants produces reactive oxygen species, causing lipid membrane damage suppressing the overall growth of the plant. The Permissible standard of lead in potable water is about 0.010 mg/L according to BIS and WHO standards 5. Contamination of lead in drinking water endures adverse ill effects viz., skin irritation, nausea, lung cancer etc.,
To march ahead the above crisis the research survey had aimed to emphasize the chemically modified newspaper pulp as an alternative potential low cost adsorbent for sequestration of Pb(II) ions from aqueous matrices6. Batch studies were accomplished to measure the influence of pH, sorbent dosage, time course, initial concentration, influence of ions, co-ions impact, effect of temperature and desorption studies.
Experimental Methods
Pre-Modification of Newspaper Pulp (PMNPP)
Newspaper was collected from home, offices and waste paper mart in Coimbatore, Tamil Nadu, India. The collected materials were cut into small bits. 50g of newspaper bits was weighed and liquefied with NaHCO3 solution with continuous stirring until the formation of pulpy nature (Figure 1a). Then, the pulp was filtered and washed with doubly distilled water until pH attains 6.5-7.0. After filtration, a homogenized pulp was obtained and dried in oven. The dried pulp was pulverized using mixture grinder to impart a fibrous consistency and larger surface area than the raw newspaper.
Chemical Modification of Newspaper Pulp
The pre-modified newspaper pulp (PMNPP) was chemically modified using 0.1 N Citric Acid (CA), the pulp was completely immersed in the acid solution (Figure 1b). The modification was based on the addition of C=O from citric acid to the pre-modified pulp. Then, the addition of acidic anhydride between the C=O group by the removal of water leads to ester linkage between the OH molecule of cellulose and anhydride. The mixture of CA and NPP were agitated at 120 rpm for 90 minutes in Kemi Oribtal mechanical shaker. After agitation, the mixture was then washed with water to remove the excess acidic nature. The Citric Acid Modified Newspaper Pulp (CAMNPP) was dried and pulverized to procure the same consistency as the PMNPP. Only the modified Newspaper Pulp sorbent material (CAMNPP) was employed for further experiments.
 |
Figure 1 (a). Pre-Modification of NPP. (b). Chemical-Modification of NPP. |
Preparation of Stock solution
A stock solution of 1000 mg/L of Pb(II)was prepared by dissolving 1.5985 g of Lead Nitrate [Pb(No3)2] in doubly distilled water. Standard solution of 500 mg/L, 250 mg/L and 100 mg/L was prepared from the stock solution for further studies.
Pilot Study-Batch Equilibration Technique
The sorption experiments were accomplished in a batch process at room temperature. 0.25g, 0.50g, 0.75g and 1.0g of the sorbent (CAMNPP) was added to 50mL of adsorbate with an initial concentration of 100 mg/L, 250 mg/L, 500 mg/L and 1000 mg/L of Pb(II) aqueous solution. The solutions were agitated in Kemi Orbital mechanical shaker at 120 rpm for a definite period of 5 minutes time interval. Sorption parameters such as adsorbent dosage, time course and pH were enhanced by the process of continuous variation. The contents were then filtered and Pb(II) concentration was measured before and after adsorption (Residual concentration) using UV-Visible Spectrophotometer (LABINDIA) instrument.
Result and Discussion
Adsorption Studies Using CAMNPP
The CAMNPP of dosages 0.25g, 0.50g, 0.75g and 1.0g were employed as the adsorbent in the sorption studies of Pb(II) ions. The discussions of the experimental results are presented below.
Batch Equilibrium Studies
Adsorbent dosage onto sorption
The active sites availability for the uptake of sorbate ions depends on the addition of varying doses of sorbents. The influence of dosage disparity (viz., 0.25 g, 0.50 g, 0.75 g & 1.0 g) for the Pb(II) – CAMNPP experimented system is depicted in figure 2. A steep inclination was observed in the curve of CAMNPP up to 100 mg/L beyond which a saturation point is reached at higher concentrations, where maximized curve is seen for 1.0 g dose 8. The dosage of the adsorbent material determined has accounted for the maximum removal of sorbate, this was taken as the optimum condition for further sorption studies.
 |
Figure 2: Effect of adsorbent dosage. |
Initial Concentration and Time Course onto sorption
The impact of Pb(II) initial concentration and time course schemes at present conditions for CAMNPP was depicted in figures 3. It reveals consistent increments in the amounts of Pb(II) adsorbed for 100 mg/L by the display of smooth plateau. Attainment of an stability state is emphasized at 100 mg/L Pb(II) concentration at 5 minutes time course. It suggests the possibility of monolayer coverage upon CAMNPP. Also, reduced amounts of Pb(II) adsorbed at concentration higher than 100 mg/L imply the availability of limited sorption sites ratio between sorbent-sorbate species [9]. Henceforth, a minimum period of 5 minutes and 100 mg/L initial concentration have been chosen as the optimized conditions for Pb(II)-CAMNPP system. The deduction efficacy of Pb(II) by CAMNPP enlarged due to the lavish accessibility of active sites on the sorbent material.
 |
Figure 3: Initial Concentration and Time course onto sorption. |
pH onto sorption
Approximate parabolic curves for the impact of pH onto Pb(II) – CAMNPP system is depicted in figure 4. Maximum percentage removal (75%) had occurred at pH 5.0 followed by a dip in the curve. This is supported by the findings of N. M.Andal et al.,1 where less sorption at acidic pH show that H+ ions compete to get adsorbed ahead of Pb2+ ions. Similarly, diminished sorption at alkaline pH support the complex formation of Pb2+ ions with hydroxyl ions.
 |
Figure 4: pH onto sorption. |
Effect of Cations
The influence of sodium and potassium ions on the Pb(II)-CAMNPP system from the resulting adsorption experiments is shown in figure 5. The reduction in the deduction of Pb2+ ions at different K+ ion environment implies a gradual decrease in the rate of Pb2+ ions getting adsorbed over the surface of the CAMNPP, as compared to that of Na+ ion environment matrices. The effect of K+ ion in reducing the adsorption of lead(II) ion is greater when compared to that of Na+ ion. This is because smaller the size of an ion, greater the degree of its hydration, as supported with D. Harikishore Kumar Reddy et al., 10 As Na+ ion is smaller in size its hydration is more effective and its inhibition for the Pb(II) uptake by CAMNPP is less in comparison with K+ ion interference.
 |
Figure 5: Impact of Cations. |
Effect of Anions
The sorption studies were accomplished in the presence of two different anions Viz., Carbonate (CO32−) and Sulphate (SO42−) along with Pb(II) ions in different concentrations and the results were potted in Figure 6. The outcomes instance that the adsorption capacity of Pb(II)-CAMNPP system was reduced with escalation in concentration of anions. The presence of CO32− and SO42− anions influenced the ability of Pb(II) ions in surface matrices. Hence, desired anions have a durable obstruct effect and CAMNPP material has more attraction towards highly charged anions.
 |
Figure 6: Impact of Anion. |
Effect of Co-ions
The result of the analysis at the influence of Co-ions (Viz., Zinc and Nickel) reveals that the presence of the foreign ions diminished the sorption rate (Figure 7). The effects of Co-ions of varying concentrations ranging from 0-100 mg/L shows, it is obvious that the percentage of Pb(II) removed by CAMNPP (75 %) in the absence of other ions was found to reduce nearly 69.43 % in the presence of Zn(II). The presence of Ni(II) as a Co-ion reduced the presence of Pb(II) to 70.51%.
 |
Figure 7: Impart of Co-ions. |
Temperature onto sorption
The temperature (293-323 K) on Pb(II) sorption using CAMNPP (Figure 8) initiated a shoot up in the Pb(II) removal at 303K, beyond which an increment was observed at higher temperatures due to flexibility of adsorbate species aiding to a sorption improvisation. The rise in numerous active sites availability for sorption process leads to the swelling of sorbent material, so that there might be a decline in the boundary layer thickness on all the sides of the chosen material. Similar results have been observed by Nisha Gaur et al., 11 which shows the mobility of metal ions increases at higher temperature.
 |
Figure 8: Effect of Temperature. |
Desorption of Pb(II)
Desorption studies probes the possibility of reprocessing the loaded adsorbents and regenerating the same for further sorption process. Studies were carried out for Pb(II)-CAMNPP systems to assess the efficiencies of the packed sorbents by employing various strengths of HCl. Figure 9 indicates that the percentage of desorption was inversely proportional to the concentration of HCL vicinity.
 |
Figure 9: Desorption of Pb(II). |
Material Screening
FTIR Spectroscopic Analysis (SHIMADZU)
The FTIR spectra of CAMNPP and Pb(II) loaded NPP lies between 4000-400 cm-1 characteristic region providing cellulose peaks at 1056.99 cm-1 and 1026.13 cm-1. The spectral bands 1103.28 cm-1 and 1157.29 cm-1 also corresponds to cellulose (Mardiah et al., [14]). In FTIR spectra of CAMNPP (Figure 10), the band at 1689.64 cm-1 resembles the active vibrations of fibrous sorbent material due to the absorption of water molecule. The spectral band 1743.65 cm-1 indicates the ester linkage between NPP and Citric acid. In FTIR spectra of Pb(II) loaded NPP (Figure 11) , the extent of band shifting in the region of 1026.13 cm-1 indicates the point of interface between functional groups and Pb2+ ions. After adsorption, the intensity of peaks becomes reduced and stipulated the involvement of metals over the surface of the sorbent material.
 |
Figure 10: FTIR – CAMNPP. |
 |
Figure 11: FTIR – Pb(II) loaded CAMNPP. |
SEM Micrograph Analysis (JEOL)
The morphology of Raw NPP portrayed in the figure 12, discloses the presence of irregular fibers, which are arranged randomly in horizontal and vertical directions with plenty of adhesives and ink. The microstructure of PMNPP (Figure 13) consists of fine pores which are filled by agglomerated fibers and alkali metal ions. The surface of CAMNPP (Figure 14) consists of less flocs with more consistent than PMNPP. After CA modification, the alkali’s presents on the fibrous materials are diminished. The pores are opened for the entry of contaminant particles and thus CAMNPP helped in sorption process. After sorption process, the metal Pb(II) deposited on the fibrous surface of CAMNPP exposed in SEM image (Figure 15). Interaction with Pb(II) indicates the presence of patches over the surface through Vander Waal’s force (Physisorption).
 |
Figure 12: Raw NPP. |
 |
Figure 13: PMNPP. |
 |
Figure 14: CAMNPP. |
 |
Figure 15: Pb(II) loaded CAMNPP. |
EDAX Spectrum Elemental Analysis (JEOL)
The EDAX spectrum depicted in Figure. 16 of raw NPP reveal the existence of the main elements such as Ca, O and Si whereas, the material PMNPP Figure 17 in contains C, O and inorganic salts such as Ca, K, Si, Al. Similarly, the material CAMNPP (Figure 18) contains C, O, Ca, Si and Al. The spectrum of Pb loaded NPP (Figure 19) consists of O, Ca, Al and Si. Cellulose, a main component of newspaper is primarily composed of C and O. The others elements such as inorganic salts exist from stuffs the added by paper mills.
 |
Figure 16: EDAX spectrum of Raw NPP. |
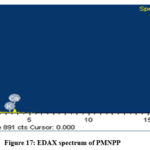 |
Figure 17: EDAX spectrum of PMNPP. |
 |
Figure 18: EDAX spectrum of CAMNPP. |
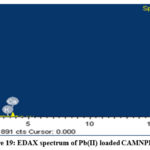 |
Figure 19: EDAX spectrum of Pb(II) loaded CAMNPP. |
Adsorption Isothermal Studies
Adsorption isotherm is a key domain to determine the adsorption process. Different adsorption isotherm models viz., Langmuir, Freundlich and Temkin have been deliberated for the verified system.
Langmuir Model
The Langmuir plot of Ce /qe vs Ce is described in Figure 20. The Langmuir constants qm and n are obtained from the the plot (Table.1) From the correlation coefficient (R2) value (0.999), it is evident that Langmuir adsorption model fitted with the adsorption of Pb(II) – CAMNPP sorbent system. A uniform adsorption occurs between Pb2+ ions and CAMNPP onto single molecular layer scheme. In accordance, all the RL values presented in Table. 2 are less than zero. Hence, the deduction of lead is favourable at different desired concentrations.
 |
Figure 20: Langmuir Plot |
Table 1: Isothermal Constants.
|
System |
Langmuir |
Freundlich |
Temkin |
||||||
|
qm (mg/g) |
b (L/g) |
R2 |
KF (mg/g) |
1/n |
R2 |
AT (Lg-1) |
B (Jmol-1) |
R2 |
|
|
Pb(II) – CAMNPP |
58.13 |
1.33 |
0.999 |
87.45 |
-0.0648 |
0.9317 |
0.864 |
-4.202 |
0.929 |
Freundlich Model
The Freundlich constants Kf and n are attained from the the plot log qe vs log Ce as (Figure 21). The obtained 1/n value indicate the interface between the CAMNPP and Pb(II). The factors of the Freundlich isotherm (Table 1) indicate that the model has fitted well according to the correlation coefficient (R2). Even though 1/n values are less than unity, lower R2 values are obvious than Langmuir. Hence, Freundlich model is less favored by the system.
 |
Figure 21: Freundlich Plot. |
Temkin Model
The plot of qe vs ln Ce representing the Temkin model was shown in Figure. 22. The factors and the corresponding R2 value, were listed in Table 1. It is found that the Temkin adsorption model fitted with Pb(II) – CAMNPP system which shows a uniform distribution of adsorbate onto adsorbent surface. The R2 value is small compared to Langmuir and Feundlich adsorption isotherms.
 |
Figure 22: Temkin Plot. |
Evaluation of R2 Factor
The correlation coefficient (R2) of all the three isotherms Viz., Langmuir (0.9991), Freundlich (0.9317) and Temkin (0.9297) are fitted well with Pb(II) – CAMNPP system. According to R2 value, the order of adsorption isotherm models obtained as Langmuir > Freundlich > Tempkin. Similary, the sorption study of M.K. Monda et al., 17 utilizing activated tea waste. It is evident that the Langmuir model describes the best sorption coverage onto Pb(II) – CAMNPP system .
Adsorption Dynamics
DH0 and DS0 calculated from Van’t Hoff ’s plots (Figure 23) are presented in table 3. Nature of feasibility, spontaneity and exothermicity are arrived from the negative values of DG0 and DH0. Positive DS0 values show the intensification of randomness at the sorption interface. Similar trends were also observed by S.Tasar et al., 20
 |
Figure 23: Van’t Hoff’s Plot. |
Table 2: Equilibrium parameter (RL)
|
Desired Concentrations (mg/L) |
Pb(II)-CAMNPP |
|
100 |
0.077 |
|
250 |
0.034 |
|
500 |
0.017 |
|
1000 |
0.008 |
Table 3: Thermodynamic Parameters.
|
Temp. (K) |
Pb(II)-CAMNPP |
||
|
∆G°X 10-3 (kJ/mol) |
∆H° (kJ/mol) |
∆S° (J/mol K) |
|
|
293 |
-0.268 |
– 4.923 |
17.86 |
|
303 |
-0.394 |
||
|
313 |
-0.549 |
||
|
323 |
-0.714 |
||
Conclusion
Citric Acid Modified Newspaper Pulp (CAMNPP) was utilized as a sorbent to sequestrate Pb2+ ions from aqueous environs. The characterization of adsorbents was evaluated by FTIR, SEM and EDAX assay. The studies indicate that a maximum of Pb(II) adsorption capacity was achieved by 75% sequestration with an adsorbent dosage of 1.0g and time course of 5 minutes at optimum pH 5.0 with an initial concentration of 100mg/L. The adsorption of the metal ion was significantly influenced by the presence of cations, anions, and co-ions. The Langmuir model fitted well with adsorption equilibrium data. The nature of feasibility, spontaneity and exothermicity was confirmed by adsorption dynamic data. It is evident that CAMNPP was found to be suitable and effective adsorbent with higher chelating ability in the removal of Pb(II) ions.
Acknowledgement
We gratefully acknowledge to the Department of Chemistry, PSGR Krishnammal College for Women for providing all facilities during our experimental works.
Conflicts of Interest
We have no conflict of interest.
Funding Sources
There is no funding source.
References
- Shyamala Devi, N; Muthulakshmi Andal, N; Vivithabharathi, K; orient. j. chem, 2018, 34, 352-361.
CrossRef - Chakraborty,R; Asthana,A; Singh,A.K; Jain, B; Hasan Susan, A.B; Int. J. Environ. Anal. Chem,2020, 102, 342-379.
- Hegazi, H.A; HBRC Journal, 2013, 9, 276-282.
CrossRef - Alli Shaikh, T.M; Biointerface Res. Appl. Chem, 2020, 10, 6522 – 6539.
CrossRef - Kaushal, A; Singh, A.K; Int J Hydro, 2017, 1(2), 1-11.
- Moyib, O. K; Ayedun , M. A; Awokoya , O. J; Omotola, O. E, Niger. J. Chem. Res, 2017, 22, 29-39.
CrossRef - Rahaman, Md. H; Islam, Md. A; Islam Md. M; Rahman, Md. A; Nur Alam S.M; Curr. Res. Green. Sustain. Chem, 2021, 4, 1-8.
- Bagali, S.S; Gowrishankar, B.S; Roy, A.S; Engineering, 2017, 3, 409–415.
CrossRef - Sai Seetha Rama Raju, D; Nageswara Rao, V; Rajendra Prasad, P; Chitti Babu, N; Int. J. Eng. Sci. Technol, 2012, 2, 1577 – 1581.
- Harikishore Kumar Reddy, D; Seshaiah, K; Reddy, A.V.R; Madhava Rao, M.; Wang, M.C; Mater, J.H; 2010, 174, 831-838.
CrossRef - Gaur, N; Kukreja, A; Yadav, M; Tiwari, A; Appl. Water Sci, 2018, 4(4), 75-81.
- Khalifaa, R.E; Abd-Eldayemb, S.N; Abd-Elmageedb, M.H; Tamera, T.M; Omera, A.M; Mohy Eldin, M.S; Desalination Water Treat, 2021, 229, 167-183.
CrossRef - Chen, H; Meng, Y; Jia, S; Hua, W; Cheng, Y; Lu, J; Wang, H; J.Pre-proof, 2020.
- Mardiah; Fathoni, R; Pudyaningtyas, P; Gamu, H; Rinaldy; MATEC Web of Conferences, 2018, 156, 1-5.
CrossRef - Mishr ; Biointerface Res. Appl. Chem, 2022, 12, 1884 – 1898.
CrossRef - Ghorbani,M; Seyedin, O; Aghamohammadhassan, M; J. Environ. Manage, 2020, 254, 1-15.
CrossRef - Monda, M.K; Korean J. Chem. Eng., 2010, 27(1), 144-151.
CrossRef - Varghese, A.G; Paul, S.A; Latha, M.S; Environ. Chem. Lett, 2019, 17, 867–877.
CrossRef - Abdelhamid, H.N; Mathew,A.P; Front. Chem. Eng, 2021, 3, 1-23.
CrossRef - Tasar, S; Kaya, F; Ozer, A; J. Environ. Chem. Eng, 2014, 2, 1018-1026.

This work is licensed under a Creative Commons Attribution 4.0 International License.










