In-Vitro pharmacological Activities of Delonix Elata Extract Mediated Zinc Oxide Nanoparticles
A. Agila1* , G. Dayana Jeyaleela2
, G. Dayana Jeyaleela2 , J. Rosaline Vimala1
, J. Rosaline Vimala1 , M. Stella Bharathy1
, M. Stella Bharathy1 , S. Margrat Sheela1
, S. Margrat Sheela1
1Department of Chemistry, Holy Cross College (Autonomous), Affiliated to Bharathidasan University, Tiruchirappalli-620 002, Tamil Nadu, India.
2Department of Chemistry, Shrimati Indra Gandhi College, Affiliated to Bharathidasan University, Tiruchirappalli-620 002, Tamil Nadu, India.
Corresponding Author E-mail: agilasrinath@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380428
Article Received on : 28 Jul 2022
Article Accepted on : 25 Aug 2022
Article Published : 29 Aug 2022
Reviewed by: Dr. Naveen Kumar
Second Review by: Dr. Jatin Mehta
Final Approval by: Dr. Faiz Bin Arith
Bio resource based metal oxide nanoparticles has potential biomedical applications. In recent years lot of research is concentrated on the production of semiconductor ZnO nanoparticles through a greener approach. The present study is focused on the biosynthesis of ZnO nanoparticles from the ethanolic extract of Delonix Elata leaves. The preliminary phytochemical screening analysis was carried out for the ethanolic extract of Delonix Elata leaves. The biosynthesized zinc oxide nanoparticles were characterized using modern analytical techniques such as UV-Visible spectroscopy, Fourier Transform Infra Red Spectroscopy (FTIR), X-Ray Diffraction Analysis (XRD), Scanning Electron Microscopy Analysis (SEM) and Energy Dispersive X-Ray Analysis (EDAX). The antioxidant potential of the synthesized zinc oxide nanoparticles are investigated by DPPH free radical scavenging assay and anti-inflammatory activity by bovine serum denaturation assay. The outcome of the studies clearly showed that the zinc oxide nanoparticles synthesized from the ethanolic extract of Delonix Elata leaves have potential anti-oxidant and anti-inflammatory properties.
KEYWORDS:Anti-oxidant; Anti-inflammatory; Delonix Elata; SEM; XRD; ZnO Nps
Download this article as:| Copy the following to cite this article: Agila A, Jeyaleela G. D, Vimala J. R, Bharathy M. S, Sheela S. M. In-Vitro pharmacological Activities of Delonix Elata Extract Mediated Zinc Oxide Nanoparticles Orient J Chem 2022;38(4). |
| Copy the following to cite this URL: Agila A, Jeyaleela G. D, Vimala J. R, Bharathy M. S, Sheela S. M. In-Vitro pharmacological Activities of Delonix Elata Extract Mediated Zinc Oxide Nanoparticles Orient J Chem 2022;38(4). Available from: https://bit.ly/3Tm6UiZ |
Introduction
The field of nanotechnology is considered to be a wonder in modern science by the researchers owing to the ability of synthesizing the highly ordered nanoparticles with different size and shape which have extensive applications 1. A tremendous expansion in the field of nanoscience is reported in the past few decades. The major advantage of synthesizing the materials in the nanorange (1-100nm) is their distinctive physico-chemical properties, optical response, non-toxic nature and catalytic efficiency due to the high surface area of the nanoparticles compared to that of the bulk materials 2. Although various semiconducting metal oxides are known, Zinc oxide is one of the semiconducting nanoparticles which is easily scalable and can be produced in large quantities due to their environmentally benign non-toxic nature. Some of the exclusive applications of ZnO nanoparticles is that they can be used in medicine, cosmetics, for energy storage applications, as a catalyst for oxidation reactions as well as in nano-optical devices 3,4. The ever-fascinating properties of zinc oxide nanoparticles made researchers to design new techniques for the production of ZnO nanoparticles. Even though various methods such as sol-gel, hydrothermal and chemical vapour deposition techniques, as well as physical vapor deposition, co precipitation and electrochemical methods are available to synthesize zinc oxide particles, the literature reports show a wide range of zinc oxide nanoparticles synthesis proceeded via bioreduction methods. The disadvantage of using expensive chemicals and hazardous solvents, and also the high cost, energy consumption and technical constraints can be eliminated in the case of bio-resource mediated synthesis of ZnO. The use of green sources such as roots, leaves, stem, bark and flower extracts for the bioreduction of metal oxide precursor solution not only offers the advantage of easy fabrication of metal oxides in nanorange, but it is also acts as capping agent preventing the agglomeration of nanoparticles 5-7. Recently, plants are used a major resource for the synthesis of non-toxic ZnO Nps. Different plant sources such as Cassia fistula and Melia azadarach 8, Passiflora caerulea 9, Camellia sinensis 10, Eucalyptus globules 11, Aloe vera 12, Vitex negundo 13, Trifolium pretense 14 etc. have been reported in the biosynthesis of ZnO particles. The phytochemical constituents such as flavonoids, alkanoids, triterpenoids, catechols, polyphenols, polysaccharides and heterocyclic compounds present in the plant sources not only serve a major role of bioreduction of metal precursor but also posses capping and stabilizing ability in the production of nanoparticles 15. In this regard, the present study is focused in the plant based synthesis of zinc oxide particles using the ethanolic extract of Delonix Elata leaves. Delonix Elata otherwise commonly known as creamy peacock flower plant (or) white gul mohur (or) yellow gul mohur is a deciduous tree. It grows 5-15 metres tall with drooping branches and is found in the gardens, avenues, amenity parks in India. It belongs to the family of fabaceae. This distinct, magnificient tree is cultivated for shade, the leaves are used as medicine 16.
Materials and Methods
The leaves of Delonix Elata were collected from places nearby Ordnance Factory Tiruchirappalli(OFT).
Preparation of ethanolic extract
The collected leaves were finely ground. From that 100 grams of the plant were weighed and taken in a beaker. 70% of ethanolic solvent is added to the grounded leaves. Then using a glass rod the mixture was thoroughly mixed, covered properly with aluminum foil, and allowed to stand for 3-4 days. After that, the extract was collected using both normal and Whatmann filter paper. The filtered extract was taken for phytochemical screening analysis. The process of extraction is shown in figures-1(A), (B) & (C).
 |
Figure 1: (A) Soaked plant in ethanol (B) Filtration process and (C) Filtered plant extract Delonix Elata leaf extract for the synthesis of Zinc Oxide nanoparticles |
0.1M of Zinc acetate was weighed about 2.195 grams taken in a beaker then added 10ml of double distilled water and it was completely dissolved using a glass rod. After the ZnO was completely dissolved it was kept in the magnetic stirrer and with 30 minutes of time intervals, twenty millilitres of the extract was added. The stirrer was maintained at 800-1200 rpm at 60°C. After 3 hrs the solution was kept aside then it was centrifuged at 6000 rpm and washed using ethanol. The centrifuged nanoparticles were collected in a 100 ml beaker and dried at 80℃ for maximum purity of the ZnO nanoparticles and it was taken for further characterization techniques.
Characterization techniques
The optical response of the formed ZnO NPs is studied using UV–Visible spectroscopy (UV–Vis) (UV-2450, Shimadzu). Functional groups identification is done by Fourier transform infrared (FTIR) spectrophotometer (Perkin-Elmer 1725x). The crystalline nature of the formed nanoparticles were determined using X-ray diffractometer (Perkin-Elmer spectrum one instrument), and shape of the synthesized particles was characterized using (SEM, Zeiss ultra-60) equipped with x-ray energy dispersive (EDS) spectroscopy.
Anti-Inflammatory Activity
Bovine Serum Albumin (BSA) Denaturation Assay
The percentage of anti-inflammatory activity (%) of each extract was assessed by bovine serum albumin denaturation assay 17. The solution containing different concentrations of samples (10 – 50 µL) was added to 1 ml of 0.1% Bovine Serum Albumin (BSA) solution and incubation is done for 5 minutes at 270C. Denaturation occurs by keeping the reaction mixture at 60 ℃ in water bath for few minutes. After cooling, the turbidity was measured spectrophotometrically at 600 nm. The percentage of inhibition was calculated using the formula,

Antioxidant Activity
DPPH Assay Method
The antioxidant activity in terms of percentage was obtained by DPPH free radical assay 18. The samples are treated with the stable DPPH radical in an ethanolic or methanolic medium. About 0.3 millimolar of DPPH reagent was prepared in 1000 ml of ethanol or methanol. 5, 10, 20, 40 & 80 µl/ml of synthesized nanoparticle were was mixed with two point five milliliters of DPPH and store at cool place then allowed to react for 30 minutes. DPPH reacts with an phyto-compounds of the leaf extracts (which can donate hydrogen) reduces the DPPH and changes its color from deep violet to light yellow. At thirty minutes, the Ab was recorded at 517nm and the radical scavenging activity (%) (i.e) anti-oxidant activity was calculated. Control reading was taken by mixing one milliliter of solvent with 2.5ml of DPPH reagent.

Ab of control = Control Absorbance
Ab of test = Test solution Absorbance
The IC50 values were calculated, the abscissa represented the concentration of the sample and the ordinate represents the average percent of radical scavenging activity.
Results and Discussion
Qualitative analysis of the Delonix elata leaf extract
In qualitative analysis, the presence of phytochemical constituents was examined from the synthesized ZnO nanoparticles. The identification of color changes denotes the presence of several phytochemical constituents. Alkaloids, Carbohydrates, Saponins, Protein, Fixed oils & fat, Phenolic compounds, Flavonoids, Terpenoids, Anthocyanin, Beta-cyanin here the above-listed phytochemicals show the positive results in qualitative analysis.
Table 1: Results of Qualitative analysis of the Delonix elata leaf extract
|
1 |
Alkaloids |
+ |
|
2 |
Carbohydrates |
+ |
|
3 |
Glucose |
– |
|
4 |
Saponins |
+ |
|
5 |
Protein |
+ |
|
6 |
Amino acids |
– |
|
7 |
Fixed oils & fat |
+ |
|
8 |
Phenolic compounds |
+ |
|
9 |
Flavonoids |
+ |
|
10 |
Terpenoids |
+ |
|
11 |
Lignins |
– |
|
12 |
Anthocyanin |
+ |
|
13 |
Beta-cyanin |
+ |
(+) = presence of phytochemicals (-) = absence of phytochemicals
 |
Figure 2: Phytochemical analysis of Delonix Elata extract. |
Visual Observation
Bio-reduction of Zinc precursor solution to zinc oxide particles using the plant extract of ethanolic extract of Delonix Elata leaves could be followed by a colour change which shows the formation of ZnO Nps. The formation of a white precipitate from the colourless ‘Zn’ precursor solution after the addition of plant extract is shown in figures 3(A), (B), (C), (D), and (E). This indicates the reduction of Zn2+ ion to Zn0 nanoparticles 19,20.
 |
Figure 3: (A) Addition of precursor (B) First addition of plant extract (C) Final addition of the plant (D) After centrifuge and (E) Liquid nanoparticles. |
UV-Visible Spectroscopy Analysis
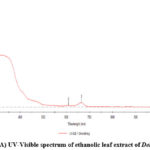 |
Figure 4: (A) UV-Visible spectrum of ethanolic leaf extract of Delonix Elata. |
 |
Figure 4: (B) UV-Visible absorption spectra of the synthesized ZnO nanoparticles. |
The optical response of zinc oxide nanoparticles is monitored with UV-Visible spectroscopy. The absorption wavelength of zinc oxide nanoparticles is reported to be between 300-450 nm 21-23. The UV-Visible absorption spectrum of extract as well as ZnO nanoparticles is shown in the figures 4(A) and (B). The ethanolic extract of Delonix Elata leaves showed three absorption peaks at 247nm, 608nm and 644nm and the synthesized nanoparticles showed an absorption band at 360 nm which indicates that the bioreduction of Zn2+ ion to Zn0 nanoparticles. The band gap (optical) of synthesized nanoparticles were found to be 3.63eV.
Fourier Transform Infra-red Spectroscopy
The organic functional groups present in the leaf extract and metal oxygen bond in the synthesized ZnO Nps are determined using the FTIR spectroscopy. For the leaf extract the bands (figure-5(A)) centered on 3600-3400 cm-1 which is due to the –O-H stretching vibrations of alcohols or phenols. The bands at around 2900 cm-1 are due to the –N-H stretching vibrations of aldehydes. The bands ranging from 1600-1510 cm-1 are due to the carbonyl stretching in proteins and those observed between1400-1000 cm-1 are due to the methylene groups from the proteins in the solution and –C-N stretching vibrations of amines. The band ranging from 879-436 cm-1 are due to the presence of ether and esters having carboxylic acid and aromatic –C-H bending. Bio-synthesized ZnO Nps reveals characteristic absorption bands (figure-5(B)) at 3407 cm-1 corresponds to the –O-H stretching of alcohols, phenols or due to the adsorbed water molecules on ZnO Nps. The bands at around 2977-2901 cm-1 are due to the –N-H stretching vibrations of aldehydes. The bands ranging from 1647 cm-1 indicate -CO stretching of proteins and those observed between1451-1047 cm-1 are due to the methylene groups and –C-N stretching vibrations of amines. The band ranging from 880-670 cm-1 ascribes to the formation of Zn-O bonds in the synthesized material. The organic functional groups observed in the FTIR spectrum for the ZnO Nps strongly suggests that the functional groups of the primary and secondary phytoconstituents of the leaf extract play the role of capping agent around the zinc oxide nanoparticles 24-25.
 |
Figure 5: (A). FTIR spectrum of ethanolic leaf extract of Delonix Elata. |
 |
Figure 5: (B). FTIR spectrum of the synthesized ZnO nanoparticles. |
X-Ray Diffraction Analysis
 |
Figure 6: XRD pattern of the synthesized ZnO nanoparticles. Click here to View figure |
Table 2: XRD data of green synthesized zinc oxide nanoparticles.
|
Pos. [°2Th.] |
Height [cts] |
FWHM Left [°2Th.] |
d-spacing [Å] |
Rel. Int. [%] |
Particle Size (nm) |
|
24.7974 |
9.30 |
3.9360 |
3.59055 |
17.33 |
2.16 |
|
31.3331 |
53.66 |
0.6216 |
2.85492 |
100.00 |
13.87 |
|
35.6520 |
41.41 |
0.7872 |
2.51836 |
77.17 |
11.08 |
|
56.4537 |
13.72 |
3.1488 |
1.63001 |
25.58 |
2.99 |
|
68.3129 |
9.27 |
2.3616 |
1.37311 |
17.28 |
4.25 |
|
Average Particle Size |
6.87 |
||||
The synthesized ZnO nanoparticle was subjected to powder-XRD technique which is useful to understand the phase and crystallinity of the nanoparticles. The peaks at 2θ positions 24.79o, 31.33o, 35.6o, 56.45o, 68o confirms the hexagonal wurtzite phase of zinc oxide nanoparticles which are shown in figure-6 and table-2. Almost identical results were also predicted by sharmila devi et al.26 in the formation of zinc oxide nanoparticles using Hibiscus rosa-sinensis leaf extract as reductant. Further the absence of peaks in XRD pattern of the zinc oxide nanoparticles are due to the amorphous nature of phytoconstituents present around the zinc oxide nanoparticles. The average size of the ZnONPs were found to be 6.87nm
Scanning Electron Microscopy Analysis
The morphological characteristics of the synthesized zinc oxide nanoparticles are observed from the scanning electron microscopy analysis. The SEM micrographs of the synthesized ZnO nanoparticles are shown in the figure-7. The synthesized zinc oxide nanoparticles possess a moderately spherical shape which is evidenced from the SEM images.
 |
Figure 7: SEM image of the synthesized ZnO nanoparticles. |
Energy Dispersive X-Ray Analysis
The composition of elements in the synthesized material is determined from the energy dispersive X-ray analysis. The EDAX spectrum of the synthesized ZnO nanoparticles is shown in the figure-8. The presence of the elements such as ‘Zn’ and ‘O’ in major composition without any other impurities confirms the presence of ZnONps.
 |
Figure 8: EDAX image of the synthesized ZnO nanoparticles. |
Anti-inflammatory Activity
Table 3: Anti-inflammatory activity of thesynthesized ZnO nanoparticles.
|
Volume of sample (µl/ml) |
Absorbance at 600 nm |
Percentage inhibition |
||||
|
DE-ZnO |
AA-ZnO |
Standard diclofenac sodium |
DE-ZnO |
AA-ZnO |
Standard diclofenac sodium |
|
|
10 |
1.019 |
1.049 |
1.010 |
06.255 |
03.495 |
07.083 |
|
20 |
0.882 |
0.965 |
0.863 |
18.859 |
11.223 |
20.607 |
|
30 |
0.713 |
0.842 |
0.701 |
34.406 |
22.539 |
35.510 |
|
40 |
0.488 |
0.529 |
0.460 |
55.105 |
51.333 |
57.681 |
|
50 |
0.243 |
0.414 |
0.275 |
77.644 |
61.913 |
74.701 |
|
IC50 Value |
36.4495 µl/ml |
42.6791 µl/ml |
36.3163 µl/ml |
|||
 |
Figure 9: Percentage inhibition graph for the bovine serum denaturation assay using synthesized ZnO nanoparticles. |
 |
Figure 10: The bovine serum denaturation assay result on synthesized ZnO nanoparticles. |
Zinc oxide are widely synthesized and investigated for their potential anti-inflammatory property (figure-9&10). The high surface area with distinctive surface reactive properties of ZnO Nps helps in the interaction of the metal ion with the cell membrane and thereby easy dissolution of these metal ions within the membrane. The in-vitro anti-inflammatory activity was studied for the bio-synthesized ZnO Nps through bovine serum denaturation assay. The percentage inhibition increased from 06.25% to 77% on increasing the concentration of DE-ZnO Nps from 10-50(µl/ml). On the other hand the percentage inhibition observed for AA-ZnO was found to be 61.9% and that of standard Diclofenac sodium it was found to be 74.7%. The IC50 value for DE-ZnO, AA-ZnO and for standard Diclofenac sodium was 36.44 µl/ml, 42.67 µl/ml and 36.31 µl/ml respectively. It is concluded that the formed ZnO Nps showed good anti-inflammatory property which can be attributed to the fact that the Zn2+ ions released by the dissolution of ZnO Nps penetrates, blocks the pro-inflammatory cytokines, interleukin in the mast cells and thereby inhibits the proliferation of the mast cells27-29.
Investigation of Anti-oxidant property of ZnO Nps
Table 4: Anti-oxidant activity of the synthesized ZnO nanoparticles.
|
S.No |
Concentration (µl/ml) |
Standard Absorbance |
Sample Absorbance (DE – ZnO) |
Standard % of DPPH Scavenged |
Sample % of DPPH Scavenged (DE – ZnO) |
|
1 |
5 |
0.539 |
0. 184 |
0.554 |
25.506 |
|
2 |
10 |
0.411 |
0.167 |
14.169 |
32.389 |
|
3 |
20 |
0.284 |
0.141 |
27.601 |
42.915 |
|
4 |
40 |
0.198 |
0.102 |
53.468 |
58.704 |
|
5 |
80 |
0.078 |
0.083 |
65.614 |
66.397 |
|
IC50 value |
|
37.3186 |
28.9746 |
||
 |
Figure 11: Percentage of DPPH scavenged by the ZnO Nps synthesized from the ethanolic leaf extract of Delonix Elata. |
Table 5: Anti-oxidant activity of the synthesized AA-ZnO nanoparticles.
|
S.No |
Concentration (µl/ml) |
Standard Absorbance |
Sample Absorbance (AA – ZnO) |
Standard % of DPPH Scavenged |
Sample % of DPPH Scavenged (AA- ZnO) |
|
1 |
5 |
0.539 |
0.218 |
0.554 |
11.740 |
|
2 |
10 |
0.411 |
0.197 |
14.169 |
20.242 |
|
3 |
20 |
0.284 |
0.175 |
27.601 |
29.149 |
|
4 |
40 |
0.198 |
0.149 |
53.468 |
39.676 |
|
5 |
80 |
0.078 |
0.144 |
65.614 |
41.700 |
|
IC50 value |
|
37.3186 |
90.2659 |
||
 |
Figure 12: Percentage of DPPH scavenged by the AA-ZnO Nps. |
DPPH free radical test is an easy and rapid method to investigate the antioxidant property of the zinc oxide synthesized by bioreduction method. DPPH molecule has delocalized free electron imparting a violet color. The characteristic absorption band of the DPPH molecule is centered around 515-520nm. The percentage inhibition increases from 25% to 66% on raising the concentration of ZnO from 5 (µl/ml) to 80 (µl/ml). The ZnO Nps IC50 value to quench the DPPH is observed to be 28.97% and that for the standard ascorbic acid it was found to be 37.31% (figure-11 & 12). The antioxidant property shown by the ZnO Nps can be attributed to the fact that the methanolic solution of DPPH free radical (violet colour) in its unstable form having an odd electron on the nitrogen atom gets reduced to stable DPPH molecule (yellow colour) by passing through an electron from the oxygen atom. On the other hand, the antioxidant activity exhibited by the ZnO Nps can also be attributed to their ability to donate hydrogen for DPPH free radical reduction 30-31.
Discussion
The ethanolic leaf extract of the medicinal tree Delonix Elata containing several phytoconstituents such as Alkaloids, Carbohydrates, Saponins, Protein, Fixed oils & fat, Phenolic compounds, Flavonoids, Terpenoids, Anthocyanin, Beta-cyanin act as a potential agent for the reduction and formation of ZnO Nps. The synthesized zinc oxide nanoparticles possessed were white in colour, showed an absorption peak at around 360nm. The FTIR studies confirmed the formation M-O bond in the formed material and the spherical morphology, hexagonal wurtzite phase and purity of the ZnO Nps were analyzed from the SEM, XRD and EDAX data are in consistent with the results when compared to the similar studies reported by other researchers for the reduction of zinc oxide nanoparticles. The IC50 value for the antioxidant potential of zinc oxide nanoparticles produced from the ethanolic leaf extract of Delonix Elata was 28.97µl/ml. Similarly, the antioxidant activities done by Dogganal Jayappa et al.[17] and Nethravathi et al.[32] for the bio-synthesized zinc oxide nanoparticles using the leaf extracts of Mussaenda frondosa L. and Garcinia xanthochymus were 824 µg/ml and 8000 µg/ml. The comparison of the results of antioxidant activity conducted in the present study with that of the results of antioxidant activity reported by other researchers revealed that the formation of zinc oxide nanoparticles from the ethanolic leaf extract of Delonix Elata have good potential anti-oxidant properties. The anti-inflammatory studies carried out for the green synthesized zinc oxide nanoparticles showed varying range of percentage inhibition. The DE-ZnO NPs showed 77% inhibition for 50 µl/ml of ZnO nanoparticles that is comparable with the standard drug Diclofenac sodium (74% inhibition for 50 µl/ml). Similarly, Dogganal Jayappa et al.[17] reported significant anti-inflammatory activity for the biosynthesized ZnO NPs (89.3% for 500 µg/ml) that is comparable with that of the standard Diclofenac sodium (92.16% for 500 µg/ml). Hence, the present anti-inflammatory studies evaluated for the green synthesized zinc oxide nanoparticles via bovine denaturation assay revealed the good anti-inflammatory potential of ZnO NPs.
Conclusion
The current research work reports the successful synthesis of ZnO Nps from the ethanolic leaf extract of Delonix Elata. The phytochemical screening analysis carried out for the ethanolic extract of Delonix Elata confirmed the presence of important primary and secondary metabolites. The UV-Visible spectroscopy showed a prominent absorption band centered at around 360 nm indicating the bioreduction of Zn2+ ions to Zn0 nanoparticles. From the UV-visible spectral data the band gap of Delonix Elata mediated zinc oxide material is found to be 3.63ev. The increase of energy band gap will decrease the size of the nanoparticles thus, the Delonix Elata mediated nanoparticle size is will be small which is also supported by the XRD size calculations. The involvement of primary and secondary metabolites in the bioreduction of zinc precursor solution to zinc oxide nanoparticles and their ability as capping agent is evidenced from the FTIR studies. The crystallinity of the synthesized nanoparticles is studied from the XRD studies and by using scherrer equation the size of the nanoparticle is calculated as 6.87nm. The morphology as well as the composition of the synthesized ZnO nanoparticles is determined from the SEM and EDAX analysis. The biogenically synthesized zinc oxide nanoparticles exhibited good anti-oxidant and anti-inflammatory activity. From our present research work we conclude that the zinc oxide nanoparticles produced from the Delonix Elata leaf extract has potential biological applications and can be explored for more in-vitro and in-vivo biological studies.
Acknowledgement
All the authors thank the management of Holy Cross College (Autonomous), Tiruchirappalli-2, Tamilnadu, India for giving permission to carried this research work. Also thank the CECRI, Karaikudi, Tamilnadu, India for did the spectral analysis.
Conflict of Interest
There are no conflict of interest.
Funding Sources
There is no funding source.
References
- Arruda, S.C.C.; Silva, A.L.D.; Galazzi, R.M.; Azevedo, R.A.; Arruda, M.A.Z. Nanoparticles applied to plant science: a review. Talanta, 2015, 131, 693–705.
- Makarov, V.V.; Love, A. J.; Sinitsyna, O. V.; Makarova, S. S.; Yaminsky, I. V.; Taliansky, M. E.; Kalinina, N. O. Green nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae, 2014, 6(1), 35–44.
- Sushma, N. J.; Mahitha, B.; Mallikarjuna, K.; Deva, P. R. B. Bio-inspired ZnO nanoparticles from Ocimum tenuiflorum and their invitro antioxidant activity. Appl. Phys. A, 2016,122, 544.
- Ramesh, P.; Rajendran, A.; Sundaram, M. Green synthesis of zinc oxide nanoparticles using flower extract Cassia Auriculata. J. Nanosci. Nanotechnol. 2014,2(1), 41–45.
- Al-Dhabi, N. A.; Arasu, M. V. Environmentally friendly green approach for the production of Zinc oxide nanoparticles and their anti-fungal, ovicidal, and larvicidal properties. Nanomaterials, 2018,8(7), 500.
- Shah, R.K.; Boruah, F.; Parween, N. Synthesis and characterization of ZnO nanoparticles using leaf extract of Camelia sinensis and evaluation of their antimicrobial efficacy. Int. J. Curr. Microbiol. App. Sci., 2015, 4(8), 444–450.
- Jayaseelan, C.; Abdul Rahuman, A.; Vishnu Kirthi, A. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2012, 90, 78–84.
- Minha Naseer; Usman Aslam; Bushra Khalid; Bin Chen. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial Potential. Sci. Rep, 2020, 10:9055.
- Santoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resource-Efficient Technologies, 2017, 3(4), 459–465.
- Nava, O.J.; Luque, P. A.; Gomez-Guti´errez, C. M.; Vilchis-Nestor, A.R.; Castro-Beltrán, A.; Mota-González, M.L.; Olivase, A. Influence of Camellia sinensis extract on zinc oxide nanoparticle green synthesis. J. Mol. Struct., 2017, 1134, 121–125.
- Siripireddy, B.; Mandal, B. K. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv. Powder Technol., 2017, 28 (3), 785–797.
- Thema, F.T.; Manikandan, E.; Dhlamini, M. S.; Maaza, M. Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater. Lett., 2015, 161, 124–127.
- Ambika, S.; Sundrarajan, M.; Antibacterial behaviour of Vitex negundo extract assisted ZnO nanoparticles against pathogenic bacteria. J. Photochem. Photobiol. B, Biol., 2015, 146, 52–57.
- Dobrucka, R. Długaszewska, J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol. Sci., 2016, 23 (4), 517–523.
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: biological synthesis and biomedical applications. Ceramics International, 2017, 43(1), 907–914. https://www.gbif.org/es/species/176798934
- Ali, S.S.; Morsy, R.; El-Zawawy, N.A.; Fareed, M.F.; Bedaiwy, M.Y. Synthesized zinc peroxide nanoparticles (ZnO2-NPs): a novel antimicrobial, anti-elastase, anti-keratinase, and anti-inflammatory approach toward polymicrobial burn wounds. Int. J. Nanomed., 2017, 12, 6059–6073.
- Manasa Dogganal Jayappa;· Chandrashekar Konambi Ramaiah; Masineni Allapuramaiah Pavan Kumar; Doddavenkatanna Suresh; Ashwini Prabhu; Rekha Punchappady Devasya; Sana Sheikh. Green synthesis of zinc oxide nanoparticles from the leaf, stem and in vitro grown callus of Mussaenda frondosa L.: characterization and their applications. Appl. Nanosci., 2020, 10, 3057–3074.
- Soren, S.; Kumar, S.; Mishra, S.; Jena, P.K.; Verma, S.K.; Parhi, P. Evaluation of antibacterial and antioxidant potential of the zinc oxide nanoparticles synthesized by aqueous and polyol method. Microb. Pathog., 2018, 119, 145–151.
- Yuvakkumar, R.; Suresh, J.; Joseph Nathanael, A.; Sundrarajan, M.; Hong, S.I. Novel green synthetic strategy to prepare ZnO nanocrystals using rambutan (Nephelium lappaceum L.) peel extract and its antibacterial applications. Mater. Sci. Eng. C, 2014, 41:17–27.
- Jacob, S. P.; Bharathkumar, R.; Ashwathram, G. Aspergillus Niger Mediated Synthesis of ZnO Nanoparticles and their Antimicrobial and in Vitro Anticancerous Activity. World J. Pharm. Res., 2014, 3(2), 3044-3054.
- Rajiv, P.; Rajeshwari, S.; Venckatesh, R. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its sizedependent antifungal activity against plant fungal pathogens, Spectrochim. Acta A Mol. Biomol. Spectrosc., 2013, 112, 384–387.
- Ain Samat, N.; Md Nor, R. Sol–gel synthesis of zinc oxide nanoparticle susing Citrus aurantifolia extracts, Ceram. Int., 2013, 39(1), S545–S548.
- Elumalai, K.; Velmurugan, S.; Ravi, S.; Kathiravan, V.; Ashokkumar, S. Facile eco-friendly and template free phytosynthesis of cauliflower like ZnO nanoparticles using leaf extract of Tamarindus indica (L.) and its biological evolution of antibacterial and antifungal activities. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2015, 136, 1052–1057.
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2015, 136, 864–870.
- Sharmila Devi, R.; Gayathri, R. Green Synthesis of Zinc Oxide Nanoparticles by using Hibiscus rosa-sinensis, Int. I. Curr. Eng. technol., 2014, 4 (4), 2444-244.
- Nagajyothi, P.C.; Cha, S.J.; Yang, I.J.; Sreekanth, T.V.M.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol., 2015, 146, 10–17.
- Mohammad, R.K.S.G.; Homayouni Tabrizi, M.; Ardalan, T.; Yadamani, S.; Safavi, E. Green synthesis of zinc oxide nanoparticles and evaluation of anti-angiogenesis, anti-inflammatory and cytotoxicity properties. J. Biosci. 2019, 44(2), 30.
- Liu, H.; Kang, P.; Liu, Y.; An, Y.; Hu, Y.; Jin, X.; Cao, X.; Qi, Y.; Ramesh, T.; Wang, X. Zinc oxide nanoparticles synthesised from the Vernonia amygdalina shows the anti inflammatory and antinociceptive activities in the mice model. Artif. Cells Nanomed. Biotechnol., 2020, 48(1), 1068–1078.
- Siripireddy, B.; Mandal, B.K. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv. Powder Technol. 2017, 28(3), 785–797.
- Murali, M.; Mahendra, C.; Nagabhushan; Rajashekar, N.; Sudarshana, M.S.; Raveesha, K.A.; Amruthesh, K.N. Antibacterial and antioxidant properties of biosynthesized zinc oxide nanoparticles from Ceropegia candelabrum L.—An endemic species. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2017, 179, 104–109.
- Nethravathi, P.C.; Shruthi, G.S.; Suresh, D.; Udayabhanu, N.; Nagabhushana, H.; Sharma, S.C. Garcinia xanthochymus mediated green synthesis of ZnOnanoparticles: Photoluminescence, photocatalytic and antioxidant activity studies, Ceram. Int., 2015, 41(7), 8680-8687.

This work is licensed under a Creative Commons Attribution 4.0 International License.









