A Review on Pyran Heterocyclic Compound as the Prosperous Scaffolds for Biological Sensor
Madhuri Suthar , Jasmin Kumbhani
, Jasmin Kumbhani , Keyur D. Bhatt*
, Keyur D. Bhatt*
Department of Chemistry, Faculty of Sciences, Mahsana urban Institute of sciences, Ganpat university, Kherva, Mahesana-384012.
Corresponding Author E-mail: drkdbhatt@outlook.com
DOI : http://dx.doi.org/10.13005/ojc/370602
Article Received on : 18-Sep-2021
Article Accepted on :
Article Published : 09 Nov 2021
Reviewed by: Dr. Muwafaq Rabeea

Second Review by: Dr. Amer J.Jarad
Final Approval by: Dr. Tayo aiyelabola
2H-pyrans and 4H-pyrans, which are six-membered heterocyclic compounds containing oxygen, are a class of biologically dynamic natural and synthetic products that played a key character in bioorganic chemistry and continue to pique attention. Pyrans and their analogues have a prominent position in bioorganic chemistry because of their numerous applications. This analysis explored the most recent advances, as well as the discovery of new methodologies and the diverse biological activities of pyran analogues.
KEYWORDS:Antimicrobial; Anticancer; Heterocyclic; Microwave; Multicomponent;
Download this article as:| Copy the following to cite this article: Suthar M, Kumbhani J, Bhatt K. D. A Review on Pyran Heterocyclic Compound as the Prosperous Scaffolds for Biological Sensor. Orient J Chem 2021;37(6). |
| Copy the following to cite this URL: Suthar M, Kumbhani J, Bhatt K. D. A Review on Pyran Heterocyclic Compound as the Prosperous Scaffolds for Biological Sensor. Orient J Chem 2021;37(6). Available from: https://bit.ly/3oehBoT |
Introduction
Because of its many advantages in terms of synthetic performance and product design, the multi-component reactions mechanism for the cost-effective synthesis of pharmacologically effective heterocyclic by one-pot has gotten a lot of consideration. Since it was developed as a method for rapidly accessing different complex heterocyclic since very basic structure blocks with high discrimination and molecule economy, this procedure has sparked a lot of interest in the creation of combinatorial libraries for medicine discovery.
In recent years, developing eco-friendly, greener, cleaner, and more productive methods has become a major focus for organic chemists. water has played a significant role in life cycles as both a support and a reaction medium for different organic alterations. There is a lot of room for multi-component reactions to be developed using water as a greener solvent media as an alternative of damaging organic solvents. However, the main problem with water is its solubility, since most organic reactants are not soluble. Researchers advanced other greener procedures, for example ecological environments, ionic liquid, and additional methods, to produce a greener synthesis. In today’s organic chemistry, optimizing reaction effectiveness through removing harmful substances, reducing unexploited generation, and having this usefulness are all critical. In this favour, the usage of agro-waste resulting catalyst has recently been demonstrated, as chemical substitutes have been unique promising study areas aimed at organic chemists. Because of their recyclable properties, 2-amino-4H-pyrans have been identified as highly potent bioactive compounds used in cosmetics dyes, and stains, as well as popular agrochemicals.1
The Evolution of Heterocyclic Chemistry
Heterocyclic chemistry started in the year of the 1800s, at the same time that organic chemistry advanced. Numerous significant developments have occurred. Brugnatelli separated alloxan from uric acid in 1818.
In 1832, Dobereiner synthesized furan (and furfural) by mixing starch and sulfuric acid. In 1834, Runge used bone dry distillation to obtain pyrrole (“fiery oil”). In 1906, Friedlander developed indigo dye, paving the way for synthetic chemistry to move a substantial amount of agrarian manufacture. In 1936, Treibs synthesized chlorophyll derivatives through crude oil, demonstrating the living origins of petroleum. Chargaff’s laws 1951 illustrate the relevance of heterocyclic chemicals (purine bases as well as pyrimidines) in the inherent code.2
Synthesis of Pyrans
Farzaneh Mohamadpour published a paper on a green synthetic way for the suitable groundwork of tetrahydrobenzo pyran scaffold in aqueous/ethanol media with theophylline as a green and biobased catalyst through tandem Knoevenagel–Michael cyclocondensation. A mixture of benzaldehyde, malononitrile and dimedone is used . These reactions are fast and produce high yields of product.3(Fig-1)
 |
Figure 1: Synthesis of tetrahydrobenzo pyran derivatives. |
Arup Dutta, Noimur Rahman, and his colleagues found uric acid, which is a naturally recyclable, renewable, and environmentally benign catalyst. It was created and used to make pyran annulated heterocyclic schemes from preliminary materials in a green, modest, and profitable way. In order to create dihydropyrano chromenes, a combination of malononitrile, aromatic aldehydes, 4-hydroxycoumarin, and uric acid in ethanol was heated at 600°C for 20-35 minutes.4(Fig-2)
 |
Figure 2: Synthesis of dihydropyrano[2,3-c]pyrazoles. |
Ag/CuO/MCM-48 originated as an active catalyst aimed at the multi component synthesis of new pyranopyrazole hybrids through Fateme Tavakoli et al. In a multi component synthesis of malononitrile, 3- (1-methyl-1-H-pyrrol-2-yl) 3-oxopropanenitrile and different aromatic aldehydes, the catalytic activity of Ag/CuO/MCM-48 was studied, It is generating novel pyrrole hybrid derivatives with short reaction durations (5–10 min) with excellent products (88–97 percent).5(Fig-3)
 |
Figure 3: Synthesis of 2‐ pyranylpyrrole derivatives. |
Preeti singh et al. successfully synthesised an environmentally stable, magnetically retrievable amine-functionalized catalyst that is SiO2@Fe3O4. In multicomponent synthesis of 2- amino-4H benzopyran pyran derivatives, the catalytic proficiency of this naturally benign NH2@ SiO2@Fe3O4 catalyst was studied. At room temperature grinding numerous dimedon, substituted aromatic aldehydes, and malononitrile below solvent- and left-over environments resulted in outstanding products and high purity.6(Fig-4)
 |
Figure 4: Synthesis of 2-Amino-4-(4-bromophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H- chromene-3-carbonitrile. |
Zhang et al were reported an effective and ecological protocol established which is used for the synthesis of spirooxindole-pyran derivatives in water-ethyl lactate using a multi component reaction which involves isatins, malononitrile, and 2-hydroxynaphthalene-1,4-dione, 4-hydroxycoumarin, and dimedone.7(Fig-5)
 |
Figure 5: Synthesis of spirooxindole-pyran derivatives. |
Andrey N. Komogortsev et al. developed a multi-component reaction containing 3-hydroxy-4H-pyran-4-ones, a-keto aldehydes, and active methylene nitriles were combined to provide a novel efficient one-pot technique used for the synthesis of 2-amino furans. The development of 2-amino furans, as opposed to 2-aminopyrans, is a unique aspect of the proposed system. high yields, slight reaction environments, low cost, and a simple examination method that avoids chromatographic purifications are all advantages of this synthesis.8(Fig-6)
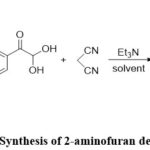 |
Figure 6: Synthesis of 2-aminofuran derivatives. |
The reaction between 4- chlorobenzaldehyde, malononitrile and 4-hydroxycoumarin in the presence of the [TMG-H] [TEA-H][(HCl)(HSO4)2] as catalyst under solvent-free condition which yields 2-amino-4-(4-chlorophenyl)-3-cyano-4H,5H-pyrano[3,2-c][1]benzopyran-5-one according to Maedeh Saeedi Mirak -Mahaleh et al have been reported.9(Fig-7)
 |
Figure 7: Synthesis of 2-amino-4-(4-chlorophenyl)-3-cyano-4H,5H-pyrano[3,2-c][1] benzopyran-5-one. |
Magnetic nanoparticles have burdened on halloysite nanotubes, an aluminosilicate clay mineral, according to Ali Maleki et al. In the synthesis of 4H-pyran derivatives, as a heterogeneous catalyst Fe3O4/HNTs was and the effectiveness was tested. The present work’s most significant advantages are its good effectiveness, slight reaction environments, green solvents, and use of an environmentally safe and recoverable catalyst. Furthermore, after seven runs, the Fe3O4/HNTs nano catalyst’s stability and efficiency were verified by easy separation and reuse.10(Fig-8)
 |
Figure 8: Synthesis of 4H-pyran derivatives using Fe3O4/HNTs nanocomposite as catalyst. |
The synthesis of pyrano[4,3] pyrans was stated by Nader Ghaffari Khaligh with a catalytic quantity of 4,40-trimethylene dipiperidine (TMDP) as a new, effective, and biodegradable organocatalyst in a ball milling process at room temperature. A Knoevenagel condensation is an important aspect of this protocol, and to our information, it is the primary article shows the catalytic effectiveness 4,40- trimethylenedipiperidine aimed at a one-pot multicomponent reaction below dissolve state environments.11(Fig-9)
 |
Figure 9: Synthesis of pyrano[4,3-b]pyran derivatives. |
Xingquan Xiong et al. have produced a modest, extremely effective, and environmentally friendly way aimed at the synthesis of 2-amino-4H-benzo pyrans and 1,4-dihydropyridines using γ-cyclodextrin as catalyst in a solvent of urea-choline chloride. In presence of a 5 mol% γ -CD catalyst, all of the reactions were approved out successfully below moderate circumstances and yielded decent to outstanding products (86–98%) in 8–28 minutes. The urea-ChCl- γ -CD catalytic process, in particular, might be transformed and recycled up to six times with just a small decrease in product yields.12(Fig-10)
 |
Figure 10: Synthesis of 2-amino-4H-benzo pyran. |
According to Zeng-Jie Yang et al. through a multi-component reaction of malononitrile, aldehyde and ethyl acetoacetate in one pot, a modest, operational, and environmentally friendly biocatalytic procedure for constructing 2-amino-4H-pyrans has been developed. Lipases’ catalytic activity was studied in various reaction media and other laboratory settings. The method described here is a gentle way to make a collection of 2-amino-4H-pyrans with high yields.13(Fig-11)
 |
Figure 11: Synthesis of 2-amino-4H-pyrans via tandem multi-component reaction. |
Saigal et al have reported new merged spiro-4H-pyran derivatives below green environments for development of agents having antimicrobial action. The synthesized molecules were primarily separated aimed at in vitro antiseptic action in contradiction of two Gram-positive and three Gram-negative microbial straining, and all the compounds revealed modest to potent antiseptic action.14(Fig-12)
 |
Figure 12: Synthesis of Spiro 4H Pyran Compound. |
Ágnes Magyar et al. recorded the excellent yields of a sequence of penta substituted 4H-pyrans and Tetrahydrobenzo pyrans via a one-pot condensation of malononitrile, aromatic aldehydes, dicarbonyl compound, an ethyl acetoacetate and acetyl-acetone or dimedone, in the existence of a four-molecular sieve changed.15(Fig-13)
 |
Figure 13: Zinc-catalyzed synthesis of 4H-pyrans. |
Yogesh B.Waghet et al. have identified a newer, more flexible, and simple synthetic technique for creating functionalized spirooxindole-pyran assisted heterocycles. At room temperature, a CsF-promoted fast tandem Knoevenagel-Michael cyclocondensation reaction of malononitrile, isatin, and 4-hydroxycoumarin is used.16(Fig-14)
 |
Figure 14: Synthesis of spirooxindole-pyran annulated heterocycles using CsF. |
The multicomponent reaction of salicylaldehydes, 2-aminopropyl-1 ene-1,1,3 tricarbonitrile, and 4-hydroxy-6-methyl-2H-pyran-2-one in a limited quantity of pyridine–ethanol solvent scheme was stated by Michail N. Elinsonet et al. which ends in the formation of the 5-(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-5H-chromeno[2,3-b]pyridine.17(Fig-15)
 |
Figure 15: Synthesis of 5-(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)- 5H-chromeno[2,3-b]pyridine. |
Unique key areas of pharmacological chemistry and green chemistry, according to Maryam Kamalzare et al, is to create simple, functional, and low-cost catalysis schemes via natural resources. Using heterogeneous bio nanocatalyst in conjunction with magnetic nanoparticles might help achieve those goals due to nanocatalyst’s ability to separate easily. Multicomponent reaction of malononitrile, aldehyde and dimedone or ethyl acetoacetate catalysed by CuFe2O4@starch at room temperature.18 (Fig-16)
 |
Figure 16: Synthesis of 2-amino 4H pyran derivatives using CuFe2O4@starch as a catalysed in ethanol. |
Galal H. Sayed et al have reported below together conventional and microwave approaches, 2-amino-4H-pyran-3-carbonitrile derivatives were synthesized and reacted by different substances. A new multicomponent one-pot reaction of 2,4-dimethoxyacetophenone, 4-methoxybenzaldehyde and malononitrile in the existence of sodium ethoxide, as a catalyst, via both conventional and microwave- assisted approaches as given in the following equation.19(Fig-17)
 |
Figure 17: Synthesis of 2-amino-4H-pyran-3-carbonitrile derivative. |
Smita P. Khare, et al have reported for the first time, using NaHCO3, an effective multicomponent synthesis of a sequence of novel 1,2,3-triazole-linked tetrahydrobenzo pyran derivatives through reaction of triazolyl aldehyde, dimedone and malononitrile. Furthermore an adsorption, distribution, metabolism and excretion (ADME) study in silico displays that the derivatives might have drug related properties used for more growth of novel therapeutic agents.20(Fig-18)
 |
Figure 18: Synthesis of 1,2,3 triazole linked tetrahydrobenzo pyran. |
Mohammad Nejati-Shendi et al have stated an effective multicomponent reaction of malononitrile, benzaldehyde and ethyl acetoacetate which yields 2-amino-3-cyano-4H-pyran. Also results were carried out over readily available and simply synthesized recyclable hollow mesoporous silica sphere (HMSS), comparable yields were encountered with better efficiency, lower cost and short reaction time.21(Fig-19)
 |
Figure 19: HMSS catalyzed synthesis of 2-amino-3-cyano-4H-pyran through the one-pot three-component reaction. |
Bipasa Halder et al. employed a distinct bio-mass following renewable feedstock to achieve the synthesis of 4H-pyran derivatives. A water extract of tamarin dus indica seed ash (WETSA) was used as a reaction media in a multicomponent reaction including carbonyl compound of C-H activated, malononitrile and aryl aldehyde which acts as a basic catalyst.22(Fig-20)
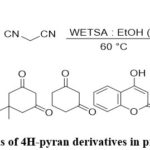 |
Figure 20: Synthesis of 4H-pyran derivatives in presence of WETSA. |
Sushama S. Kauthalea et al. reported catalyst-free multicomponent synthesis of pyran assisted heterocyclic compounds using (ethylene glycol) EG: H2O.23(Fig-21)
 |
Figure 21: EG:H2O promoted catalyst-free one-pot multicomponent synthesis of pyran annulated heterocyclic compounds. |
Conclusion
Pyran scaffolds, as previously described, can originate in an extensive range of natural materials, pharmaceuticals, and bioactive molecules. Antidiabetic, hepatoprotective, anticancer, antiatherosclerotic, geroprotective, vasodilator, bronchodilator anticancer, and antitumor properties have also been shown in molecules containing pyran scaffolds. We summarize various methods for synthesizing 4H– pyran that have been developed over the years in this study. Pyran products were synthesized in high yields. As a result, future advancements are likely to put a greater emphasis on more cost-effective and resource-friendly approaches to the development of the pyran moiety and its use in a wide range of applications.
Acknowledgement
We thank our institute Mehsana Urban Institute of Sciences, Ganpat University for providing laboratory and library facilities.
Conflicts of Interest
Conflicts of Interest The authors declare no conflict of interest.
Funding Sources
There are no funding sources
References
- Maddila SN, Maddila S, Bhaskaruni SVHS, Kerry N, Jonnalagadda SB. MnO2 on hydroxyapatite: A green heterogeneous catalyst and synthesis of pyran-carboxamide derivatives. Inorganic Chemistry Communications. 2020;112. doi:10.1016/j.inoche.2019.107706
CrossRef - Arora, P.; Arora, V.; Lamba, H. S.; Wadhwa, D. IMPORTANCE OF HETEROCYCLIC CHEMISTRY: A REVIEW. IJPSR 2012, 3 (9), 2947–2954.
- Mohammadpour, F. Synthesis of Pyran-Annulated Heterocyclic Systems Catalyzed by Theophylline as a Green and Bio-Based Catalyst. Polycyclic Aromatic Compounds 2021, 41 (1), 160–172. https://doi.org/10.1080/10406638.2019.1575246.
CrossRef - Lashkari, M.; Mohamadpour, F.; Maghsoodlou, M. T.; Heydari, R.; Hazeri, N. Uric Acid as a Naturally Biodegradable and Reusable Catalyst for the Convenient and Eco-Safe Synthesis of Biologically Active Pyran Annulated Heterocyclic Systems. Polycyclic Aromatic Compounds 2020. https://doi.org/10.1080/10406638.2020.1781205.
CrossRef - Tavakoli, F.; Mamaghani, M.; Sheykhan, M. Introduction of Ag/CuO/MCM-48 as an Efficient Catalyst for the One-Pot Synthesis of Novel Pyran-Pyrrole Hybrids. Applied Organometallic Chemistry 2019, 33 (9). https://doi.org/10.1002/aoc.5083.
CrossRef - Singh, P.; Yadav, P.; Mishra, A.; Awasthi, S. K. Green and Mechanochemical One-Pot Multicomponent Synthesis of Bioactive 2-Amino-4 H-Benzo[ b]Pyrans via Highly Efficient Amine-Functionalized SiO2@Fe3O4 Nanoparticles. ACS Omega 2020, 5 (8), 4223–4232. https://doi.org/10.1021/acsomega.9b04117.
CrossRef - Zhang, M.; Fu, Q. Y.; Gao, G.; He, H. Y.; Zhang, Y.; Wu, Y. S.; Zhang, Z. H. Catalyst-Free, Visible-Light Promoted One-Pot Synthesis of Spirooxindole-Pyran Derivatives in Aqueous Ethyl Lactate. ACS Sustainable Chemistry and Engineering 2017, 5 (7), 6175–6182. https://doi.org/10.1021/acssuschemeng.7b01102.
CrossRef - Komogortsev, A. N.; Melekhina, V. G.; Lichitsky, B. v.; Minyaev, M. E. Novel One-Pot Approach to 2-Aminofuran Derivatives via Multicomponent Reaction of 3-Hydroxy-4H-Pyran-4-Ones, α-Ketoaldehydes and Methylene Active Nitriles. Tetrahedron Letters 2020, 61 (41). https://doi.org/10.1016/j.tetlet.2020.152384.
CrossRef - Mirak-Mahaleh, M. S.; Rad-Moghadam, K. A Novel Amphipathic Low-Melting Complex Salt: An Efficient Homogeneous Catalyst for Synthesis of Pyran-Annulated Heterocyclic Scaffolds and Pyrido[2,3-d]Pyrimidines. Journal of Molecular Liquids 2020, 307. https://doi.org/10.1016/j.molliq.2020.112989.
CrossRef - Maleki, A.; Hajizadeh, Z. Magnetic Aluminosilicate Nanoclay: A Natural and Efficient Nanocatalyst for the Green Synthesis of 4H-Pyran Derivatives. Silicon 2019, 11 (6), 2789–2798. https://doi.org/10.1007/s12633-019-0069-4.
CrossRef - Khaligh, N. G.; Mihankhah, T.; Rafie Johan, M. 4,4′-Trimethylenedipiperidine (TMDP): An Efficient Organocatalyst for the Mechanosynthesis of Pyrano[4,3-b]Pyrans under Solid-State Conditions. Polycyclic Aromatic Compounds 2020, 40 (5), 1606–1615. https://doi.org/10.1080/10406638.2018.1564679.
CrossRef - Xiong, X.; Yi, C.; Liao, X.; Lai, S. An Effective One-Pot Access to 2-Amino-4H-Benzo[b]Pyrans and 1,4-Dihydropyridines via γ-Cyclodextrin-Catalyzed Multi-Component Tandem Reactions in Deep Eutectic Solvent. Catalysis Letters 2019. https://doi.org/10.1007/s10562-019-02767-x.
CrossRef - Yang, Z. J.; Gong, Q. T.; Wang, Y.; Yu, Y.; Liu, Y. H.; Wang, N.; Yu, X. Q. Biocatalytic Tandem Multicomponent Reactions for One-Pot Synthesis of 2-Amino-4H-Pyran Library and in Vitro Biological Evaluation. Molecular Catalysis 2020, 491. https://doi.org/10.1016/ j.mcat .2020. 110983.
CrossRef - Saigal; Irfan, M.; Khan, P.; Abid, M.; Khan, M. M. Design, Synthesis, and Biological Evaluation of Novel Fused Spiro-4 H-Pyran Derivatives as Bacterial Biofilm Disruptor. ACS Omega 2019, 4 (16), 16794–16807. https://doi.org/10.1021/acsomega.9b01571.
CrossRef - Magyar, Á.; Hell, Z. One-Pot, Three-Component, Selective Synthesis of the Polyfunctionalized 4H-Pyran and 4H-Benzo[b]Pyran Derivatives in the Presence of a Highly Efficient Molecular Sieve-Supported Zinc Catalyst. Green Processing and Synthesis 2018, 7 (4), 316–322. https://doi.org/10.1515/gps-2017-0083.
CrossRef - Wagh, Y. B.; Padvi, S. A.; Mahulikar, P. P.; Dalal, D. S. CsF Promoted Rapid Synthesis of Spirooxindole-Pyran Annulated Heterocycles at Room Temperature in Ethanol. Journal of Heterocyclic Chemistry 2020, 57 (3), 1101–1110. https://doi.org/10.1002/jhet.3846.
CrossRef - Elinson, M. N.; Vereshchagin, A. N.; Anisina, Y. E.; Krymov, S. K.; Fakhrutdinov, A. N.; Egorov, M. P. Selective Multicomponent ‘One-Pot’ Approach to the New 5-(4-Hydroxy-6-Methyl-2-Oxo-2H-Pyran-3-Yl)Chromeno[2,3-b]Pyridine Scaffold in Pyridine–Ethanol Catalyst/Solvent System. Monatshefte fur Chemie 2019, 150 (6), 1073–1078. https://doi.org/10.1007/s00706-019-02388-5.
CrossRef - Kamalzare, M.; Bayat, M.; Maleki, A. Green and Efficient Three-Component Synthesis of 4H-Pyran Catalysed by CuFe 2 O 4 @starch as a Magnetically Recyclable Bionanocatalyst . Royal Society Open Science 2020, 7 (7), 200385. https://doi.org/10.1098/rsos.200385.
CrossRef - Sayed, G. H.; Azab, M. E.; Anwer, K. E. Conventional and Microwave-Assisted Synthesis and Biological Activity Study of Novel Heterocycles Containing Pyran Moiety. Journal of Heterocyclic Chemistry 2019, 56 (8), 2121–2133. https://doi.org/10.1002/jhet.3606.
CrossRef - Khare, S. P.; Deshmukh, T. R.; Akolkar, S. v.; Sangshetti, J. N.; Khedkar, V. M.; Shingate, B. B. New 1,2,3-Triazole-Linked Tetrahydrobenzo[b]Pyran Derivatives: Facile Synthesis, Biological Evaluation and Molecular Docking Study. Research on Chemical Intermediates 2019, 45 (10), 5159–5182. https://doi.org/10.1007/s11164-019-03906-0.
CrossRef - Nejati-Shendi, M.; Tebyanian, H.; Zare, R.; Ayoubi-Chianeh, M.; Roshani, K.; Kassaee, M. Z.; Rashidiani, J. Hollow Mesoporous Silica Sphere (Hmss) as a Recyclable Nano-Catalyst in an Efficient One-Pot Multicomponent Synthesis of 2-Amino-3-Cyano-4h-Pyran Derivatives. Biointerface Research in Applied Chemistry 2020, 10 (6), 6640–6651. https://doi.org/10.33263/BRIAC106.66406651.
CrossRef - Halder, B.; Maity, H. S.; Banerjee, F.; Kachave, A. B.; Nag, A. Water Extract of Tamarindus Indica Seed Ash: An Agro-Waste Green Medium for One-Pot Three-Component Approach for the Synthesis of 4H-Pyran Derivatives. Polycyclic Aromatic Compounds 2020. https://doi.org/10.1080/10406638.2020.1858885.
CrossRef - Kauthale, S. S.; Tekale, S. U.; Kótai, L.; Kendrekar, P. S.; Pawar, R. P. Synthesis of Pyran Annulated Heterocyclic Compounds under Catalyst Free Conditions Using Aqueous Ethylene Glycol. Organic Preparations and Procedures International 2020. https://doi.org/10.1080/00304948. 2020.1812360.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









