Proximate Analysis, Antoxidant Property and Cytotoxicity Assessment for Pseuderanthemum Reticulatum Leaves
Department of Chemistry, Holy Cross College (Autonomous), Affiliated to Bharathidasan University, Tiruchirappalli-620002, India
Corresponding Author E-mail: msheela86@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370428
Article Received on : 05-Jul-2021
Article Accepted on : 16-Aug-2021
Article Published : 31 Aug 2021
Reviewed by: Dr. Santhi Muthuraman
Second Review by: Dr. Antony Samrot
Final Approval by: Dr. Nenad Ignjatovic
The bioactive constituents derived from plants attract the attention of researchers due to their potential applications in the medicinal field. In this regard, the proximate analysis and the cytotoxicity study of the plant materials play an important role in the phytochemical research. In the present work, estimation of total ash, moisture content, fiber content, crude protein, and carbohydrate were carried out under proximate analysis and the antioxidant activity of the anthocyanin present in the plant material was evaluated by DPPH (2,2-diphenyl-1-picrylhydrazyl) method. The separation of anthocyanin pigment from the plant material was done by paper chromatography (PC) technique and they are characterized by UV spectrum, chemical test and the Rf values obtained from paper chromatography. This study also investigated the in vitro cytotoxicity of Pseuderanthemum reticulatum leaves by means of MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide)) assay PBMC (Peripheral Blood Mononuclear Cell). The results of the proximate analysis showed that the plant material contains 7.6% of moisture content, 16.6 % of total ash, 5.6% of crude protein, 23.0% of crude fiber, 3.82% of crude fat and 23.64 % of carbohydrate. The free radical scavenging ability of the separated anthocyanin was found to be 72.58% at 10 µg/mL. The cytotoxicity investigation showed that the aqueous extract possess the IC50 value of 161.5μg/mL. The High percentage of radical scavenging activity and low toxicity of the plant suggest that it can be extensively used for the investigation of the bioactive constituents and its applications.
KEYWORDS:Cytotoxicity; DPPH; MTT assay; Proximate analysis; Pseuderanthemum reticulatum; PBMC cells;
Download this article as:| Copy the following to cite this article: Sheela S. M, Vimala J. R. Proximate Analysis, Antoxidant Property and Cytotoxicity Assessment of Pseuderanthemum Reticulatum Leaves. Orient J Chem 2021;37(4). |
| Copy the following to cite this URL: Sheela S. M, Vimala J. R. Proximate Analysis, Antoxidant Property and Cytotoxicity Assessment of Pseuderanthemum Reticulatum Leaves. Orient J Chem 2021;37(4).Available from: https://bit.ly/2WBnhPZ |
Introduction
Natural plants are used as medicine since time immemorial. They are used as direct medicinal agent or a base for many semi-synthetic chemical compounds. The chemical structures derived from the bioactive compounds are used as models for the synthesis of new compounds. Most of the natural plants are also used as sources for dietary supplements which are called phytopharmaceuticals and are produced from fresh or dried plants or parts of plants1. Medicinal plants place a significant role in both humans and animals health system not only in diseased conditions but also served as a potential material for proper maintaining of health. Qualitative and quantitative determination of the bioactive compounds from plant materials led to the discovery of new drugs and in the treatment of many diseases. The bio active compounds such as anthocyanins, phenols and flavonoids have strong anti-oxidant properties. Ajayi et.al investigated The phytochemical screening, proximate analysis, mineral composition and anti-microbial activity of Megaphrynimum macrostachyum seeds and the findings showed that the plant has good nutritional and medicinal importance2. Jadid et.al studied the mineral composition, and proximate analysis of the fruit Piper retrofractum (P. retrofractum) vahl. and the quantitative determination of total alkaloids, phenols and flavonoids were also estimated. The plant contains 63.4% of carbohydrate, 11.4% of crude protein, 4.29% of total ash, 28.8% of fiber, 2.97% of total fat and it has high nutritional and phytochemical values3. Dastagir et.al studied the proximate analysis of Zygophyllaceae and Euphorbiacea family plants such as Fagonia cretica L., Tribulus terrestris L., Ricinus communis L.,.etc., The environment, habitat and time of harvest are the reasons for the variation in the proximate composition of these plants. The results of the investigation showed that the seeds of R. communis can be useful for development of biodiesel and helpful in pharmaceutical, insecticidal and food industries4. It is essential to investigate the host cell to examine the safety of any drug either natural or synthetic.
MTT is one of the common and the most frequently applied method to evaluate the cell viability. The cell viability is determined through colorimeter using tetrazolium dye. There is a liner relationship between metabolically active cells and the color produced by them and this will allow the quantification of the cell death rate accurately. This method is based on the capability of a mitochondrial dehydrogenase enzyme of viable cells to break the tertrazolium rings; due to which the pale yellow tetrazolium dye form purple colored crystals of formazan which are impermeable to the cell membranes. Thus the formazan crystals accumulated within healthy cells are dissolved by the addition of DMSO (Di Methyl Sulfoxide). The cell viability in MTT assay is connected with the quantification of formazan crystals at 540 nm and the number of viable cells is proportional to the formazan crystals formed5. Ala et.al assessed the in vitro cytotoxic activities of the aqueous, methanol and chloroform extracts of Andrographis paniculata and Aspilia africana leaves on murine cells. The percentage of cell viability values of the combined extracts at different concentrations were determined by the tetrazolium salt reduction assay (MTT). The results showed that extracts were cytotoxic to the murine cells at 500 μg/mL concentration6. Korsuwannawong et.al studied the cytotoxicity of a Thai herb on Mouse fibroblast (L929) cells using tetrazolium (MTT) and sulfo rhodamine B (SRB) assays7. Kumar et.al evaluated the in vitro cytotoxic activity of Cocculus hirsutus by MTT assay using breast cell lines (MCF-7). Antioxidant activity of the plant extracts by DPPH scavenging method was also studied. The results revealed that it shows good antioxidant property and it can act as a potential drug for breast cancer8. Pathogenesis of many diseases involves free radicals. Our body prevents these diseases by natural defense mechanism but when the free radicals and reactive oxygen spices attack our body suddenly in a larger amount then our body needs an external antioxidant supplements. In nature many plants have natural anti-oxidants and these anti-oxidants from the natural source are highly active and preferred over the synthetic one. Anthocyanins, phenolic compounds and flavonoids derived from plant have an excellent free radical scavenging activity hence they are potent anti-oxidant9. Astadi et alexamined the anti-oxidant activity of anthocyanins in Malika and Cikuray variety of black soybean seed coat by DPPH radical scavenging and LDL oxidation methods. The maximum radical scavenging capacity of Malika and Cikuray was 92.78% and 91.50% respectively10. The radical scavenging efficacy of Anthocyanins present in a range of berries was studied by Nakajima et.al. The radical scavenging activities of these berry extracts were analyzed by using DPPH. The result showed that all the extracts exhibited good anti-radical activities11. Bo Chu Wang et.al studied the anti-oxidant properties of blueberry Anthocyanins. The blueberry Anthocyanins protect the ECV-304 cells against oxidative damage caused by H2O2; Hence they are used as a colorant in food products mainly of acidic in nature and as a food source to prevent diseases arising due to the oxidative processes12.Pseuderanthemum reticulatum is an ornamental plant and used in traditional medicine to take care of fever, headache, cold and back pain. The native of the plant is Polynesia and belongs to the family of Acanthaceae.13 In the present work proximate analysis, anti-oxidant properties of Anthocyanins by DPPH method and cytotoxicity assessment of Pseuderanthemum reticulatum plant leaves are investigated. The proximate analysis, anti-oxidant potential and cytotoxicity assessment Pseuderanthemum reticulatum is an ornamental plant and used in traditional medicine to take care of fever, headache, cold and back pain was studied 13.
Experimental
Materials
Sulphuric acid, Anthrone, Glucose, Methanol, Sodium hydroxide, Sulfo-phospho-Vanillin reagent, Chloroform, Fetal Bovine Serum (FBS)(Gibco USA), RPMI medium, DMSO, 3-4,5 dimethylthiazol-2yl-2,5-diphenyl tetrazolium bromide (MTT) (Sigma USA), 1X PBS (Himedia India), tissue culture 96 well plate , 0.1mM DPPH solution, Ascorbic acid, Butanol, Acetic acid of analytical grade were used for the investigation.
Plant Material for proximate analysis
The fresh leaves of Pseuderanthemum reticulatum were shade dried for 20 days and grounded into powder using a blender and it is stored in a dry container.
Total Ash
The residue remaining after incineration is called the total ash and it is expressed in terms of percentage. For the total ash determination, a clean dry crucible was weighed and the dried plant material (2 g) was placed and weighed again. The crucible with the plant material was heated over an electric burner. After the material was charred; it was heated again in a muffle furnace to 600ºC for 2 hours. All organic matter will be burnt at this temperature leaving behind minerals and then the crucible was taken from the furnace and cooled in a desiccator to room temperature and weighed again. The total ash percentage was determined by the following formula14.
Moisture content
Moisture content of the plant material was done by weighing crucible along with the sample and drying at 1050C in an oven. Heating was continued until a steady weight was obtained. Moisture content in terms of percentage was obtained by the following formula2.
W1- Empty crucible weight, W2- Crucible weight with sample (before drying)
W3 – Crucible weight with sample (after drying)
Estimation carbohydrate (Anthrone method)
Anthranol is an active form of the Anthrone reagent. The enol tautomer of the reagent condensed with the carbohydrate furfural derivative to form a blue colour solution which is estimated calorimetrically. The furfural derivative of carbohydrate is produced by dehydration using concentrated H2SO4. For the estimation of carbohydrate, standard glucose stock solution (50 mg/mL) was prepared and diluted to different concentrations. 100 μL of each standard solutions as well as the aqueous extract of the sample were mixed with 200 μL of 75% H2SO4 and 400 μL of Anthrone reagent. After vertexing, both the sample and standard solutions were boiled at 100°C for 15 minutes then the absorbance was recorded at 578 nm using micro plate reader15,16.
Estimation of Total crude fiber
Estimation of total fiber content is one of the nutritive measurements in poultry and domestic animal feeds and it is also used to detect adulteration in the various food products both qualitatively and quantitatively. To estimate the total crude fiber, the plant material (2g) was treated with petroleum ether at 50°C to remove the fat content. After extraction, the material was dried; then the dried material was boiled with 200mL of sulphuric acid (H2SO4) for 30 minutes. After boiling the material was filtered by means of muslin cloth and followed by hot water washing till the traces of all the acids were removed. The washed material was again boiled for 30 minutes with sodium hydroxide (200mL) solution and washed with 25 mL of boiling water with 1.25% of H2SO4 followed by 25mL of ethanol and then the residues were removed and transferred into pre-weighted ashing dish (W1). The residue was heated for 2 hours at 130°C for drying and it was cooled and weighed (W2). Then the residue was ignited at 600°C for 30 minutes, after that the ashing dish was weighed (W3) again. The percentage of total fiber content in the plant material was calculated using the following formula17.
Lipid estimation by Vanillin method
The total lipid in the plant material was estimated by colorimetric Sulfo-Phospho-Vanillin method. Standard stock solution of cholesterol (5mg/mL) was prepared using the solvent mixture of chloroform and methanol in 2:1 ratio. Standard solutions of different concentration were prepared using the stock solution. The dried plant material is also dissolved in the chloroform, methanol solvent mixture and filtered and the filtrate is used for the analysis. For colorimetric analysis Both the standards and filtrate of the plant material (500 μL) was mixed with 1mL of concentrated sulfuric acid and heated at 90oC for 10 minutes, after cooling 500 μL of Sulfo-Phosphoric-Vanillin reagent was added to each solution for the colour development and the absorbance were measured at 540 nm18.
Protein Estimation by Bradford’s method
The total protein content present in the plant material was determined by Bradford’s method. For the estimation of protein Bovine serum albumin standard was prepared at different concentrations (0.04 to 0.5mg/mL). 3ml of Bradford’s reagent was added to 100 μL of each of the standard and sample solutions respectively. Then the absorbances of all the solutions were measured at a wavelength of at 595 nm after incubating them in the dark for 5 minutes 19.
MTT Assay
In vitro cytotoxicity of the aqueous extract of the plant material was tested via MTT assay by means of PBMC.( Peripheral Blood Mononuclear Cells). The PBMC cells were seeded into 96-well plate at a density of 1×105 cells/well (200 µL) and treated with RPMI media and each sample was replicated three times and the cells were incubated in a humidified incubator at 37°C for 24 h with 5% CO2. After the incubation period, 20 µL of MTT (5 mg/ml) was added into each well and again the cells were incubated for 2-4 h at 37°C until purple precipitate was obtained. In the end, the medium together with MTT was removed from the wells and washed by 200 µL of 1X PBS (Phosphate BufferedSaline) solution. The formazan crystals formed in the plate were shaken with 100 µL of DMSO for 5 min to dissolve them. The micro plate reader (Thermo Fisher Scientific, USA) was used to measure the absorbance of the formazan crystals formed in each well at the wavelength 570 nm. By using the GraphPad Prism 6.0 software (USA), the cell viability percentage and IC50 value were calculated 5.
Separation of anthocyanin pigments using Paper Chromatography
Paper chromatography is one of the basic tools for the separation of phytoconstituents. It is mainly used to separate the colored pigments from the plant materials. To separate the anthocyanins; the plant extract of the fresh leaves was prepared using acidified methanol. The extract was placed in the paper using capillary tube. The mobile phase used in the system was a mixture of butanol, acetic acid and water in 4:1:5 ratios. Once the mobile phase reaches the solvent front, the paper is taken and the solvent front is marked and allowed to dry20. The Rf values of the colored pigment spots were calculated. The colored pigment spots were cut off from the paper and dissolved in methanol with few drops of concentrated HCl. The anthocyanin pigments were confirmed by chemical test, Rf value and UV spectrum.
Antioxidant property of the anthocyanin pigments by DPPH Method
0.1 mM of DPPH solution was prepared using methanol and 100 μL of this solution was added to the separated anthocyanin samples (300 μL of each) at different concentrations and the solution mixture was shaken strongly and allowed to rest at room temperature for 30 minutes. Then the absorbance of each solution was measured at 517 nm using a UV-VIS spectrophotometer and Ascorbic acid was used as the reference. DPPH radical scavenging ability of the separated anthocyanins was estimated by the following formula 19.
Result and Discussion
Proximate analysis
The proximate analysis result of Pseuderanthemum reticulatum dry leaves are shown in Table 1. Moisture content is one of the chief factors in determination of the shelf life of any plant materials. If the moisture content of the plants is higher than 15%, they can be easily contaminated by microorganisms. The moderate moisture content (7.6%) of Pseuderanthemum reticulatum leaves showed that it could be stored for longer lime. The availability of inorganic constituents in a plant material was given by the ash content and the plant material contains 16.6% of total ash. Fat yields more calories compared to carbohydrates and proteins. Total fat content of the plant material was found to be 3.82% by Vanillin method. Carbohydrate is one of the energy source and the estimation of carbohydrate by Anthrone method showed that the plant material contains 23.64 % of carbohydrate. The protein present in the plant material was found to be 5.6% by Bradford’s method. For the digestion of food materials in the food passage of animals, crude fiber is very essential and the results showed that the plant material contains 23% of crude fiber21.
Table 1: Proximate results of the Pseuderanthemum reticulatum leaves
|
Proximate composition |
Percentage (%) of dry sample |
|
Moisture content |
07.60 |
|
Total Ash |
16.60 |
|
Crude protein |
05.60 |
|
Crude fiber |
23.00 |
|
Crude fat |
03.82 |
|
Carbohydrate |
23.64 |
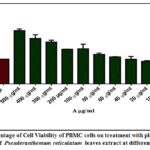 |
Figure 1: Percentage of Cell Viability of PBMC cells on treatment with plant extract (A- Concentration of Pseuderanthemum reticulatum leaves extract at different concentration) |
 |
Figure 2: Images of PBMC cells treated with plant extracts at different concentrations |
MTT Assay
MTT assay revealed the cytotoxicity of the aqueous extract of Pseuderanthemum reticulatum on PBMC cells at different concentrations. The OD (Optical Density) Values of the sampleat different concentrationand the percentage of cell viability on PBMC cells were shown in Table 2, and Figure1. The Cell viability of the extract at lower concentrations (10 to 40 μg/mL) was found between 83% to 99%.The cell viability percentage values for the concentrations from 60 μg/mL to 500 μg/mL, the result were found between 109 to 215 and the values were higher than the control. The reason for the high percentage of cell viability is due to the impact of the color of the plant extract. Since the separated anthocyanin is pink in colour during the experimental observation its absorbance has also interfered the absorbance of the test solutions in MTT assay. Images of PBMC cells treated with the plant extracts at different concentrations were shown in Figure 2 and it showed that the high concentration of the plant extract not much affect the cell viability. The overall results showed that the aqueous plant extracts have low cytotoxicity towards PBMC cells and the IC50 value was found to be 161.50 μg/mL22.
 |
Figure 3: OD Values (at 517 nm) of Anthocyanin extract at different concentrations after adding DPPH solution (Control Mean OD value: 1.198) Click here to View figure |
Table 2: OD Value (at 570 nm) of the control and samples at different concentrations
|
S. No |
Tested sample concentration (μg/mL) |
Mean OD Values
|
|
1. |
Control |
0.436 |
|
2. |
500 μg/ml |
0.937 |
|
3. |
400 μg/ml |
0.804 |
|
4. |
300 μg/ml |
0.739 |
|
5. |
200 μg/ml |
0.612 |
|
6. |
100 μg/ml |
0.619 |
|
7. |
80 μg/ml |
0.521 |
|
8. |
60 μg/ml |
0.476 |
|
9. |
40 μg/ml |
0.435 |
|
10. |
20 μg/ml |
0.399 |
|
11. |
10 μg/ml |
0.364 |
Anti-oxidant activity
Anthocyanins are very reactive towards ROS (Reactive Oxygen Species), due to their structure and their electron deficient nature. Anthocyanins and anthocyanidins are natural anti-oxidants and exhibit higher scavenging activity towards free radicals23. The coloured pigments separated from the acidified methanolic extract of Pseuderanthemum reticulatum showed a characteristic Rf values (0.42-0.78) and the UV absorption peak at 516 nm confirmed the presence of Anthocyanin pigments.24 When 2ml of HCl was added into the extract and heated for 5 minutes, the pink colour remains stable and the addition of 2ml of NaOH separately to the extract gave a green colour also indicated the presence of Anthocyanin20.The radical scavenging capacity of the separated Anthocynanins was done by DPPH method and the investigation result revealed that they have good anti-oxidant property. The free radical scavenging ability of extract was found to 77.54% and 72.58% at 500μg/mL and 10 μg/mL concentrations respectively. The concentration of the sample requested to reduce the DPPH concentration by 50% is called IC50 value. The IC50 value for radical scavenging capacity of the extract was found to be 6.94 μg/mL and it was obtained from the linear regression analysis [log (inhibitor) Vs. normalized response] using the plot of OD value Vs. Sample concentration (Figure 3). The free radical inhibition percentage of the separated Anthocyanin pigments at different concentrations was presented in Table 3.
Table 3: Free radical inhibition percentage (%) of separated anthocyanin
|
Tested sample concentration (μg/mL) |
Percentage of inhibition (%) |
|
500 |
77.54 |
|
250 |
76.76 |
|
100 |
76.29 |
|
50 |
75.87 |
|
10 |
72.58 |
|
Ascorbic acid |
92.97 |
Conclusion
Plants have been the source for traditional medicines and most of them are very useful for the development of new drugs in pharmacological industries. In the current work, proximate analysis, anti-oxidant potential and cytotoxicity of Pseuderanthemum reticulatum leaves were studied. The results of proximate analysis showed that Pseuderanthemum reticulatum could be used as a feed material for animals and the free radical scavenging ability of the separated Anthocyanis by DPPH technique revealed that it has an excellent antioxidant property. The cytotoxicity studies of the aqueous extract of the plant by MTT assay on PBMC cells showed that it exhibits low toxicity towards PBMC cells. The overall results of the investigation showed that the plant can be used in pharmacological research. With the ongoing research on this plant, new bioactive principles can be derived in the future.
Acknowledgement
The authors are grateful to the Management of Holy Cross College (Autonomous), Affiliated to Bharathidasan University, Tiruchirappalli-2, Tamil Nadu, India for their support for this work and also thankful to Trichy Research Institute of Biotechnology Pvt. Ltd. Trichirappalli, India to carry out the toxicity studies.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Reference
- Raaman, N. Phytochemical Techniques,New India Publishing Agency, New Delhi. 2006.
- Ajayi, I.A.; Ojelere, O.O. International Journal of Engineering Research & Technology, 2013, 2(9), 2123-2131.
- Jadid, N.; Arraniry, B.A.; Hidayati, D.; Purwani, K.I.; Wikanta,W.; Hartanti, S.R.; Rachman, R.Y. Asian Pac J Trop Biome., 2018, 8(1), 37-43.
CrossRef - Dastagir, G.; Hussain, F.; Khattak, F.; Khanzadi.;Sarhad, J. Agric., 2013 29(3), 395-400.
- Bahuguna, A.; Khan, I.; Bajpai, V.; Kang, S. Bangladesh Journal of Pharmacology.,2017, 12( 2), 115-118.
CrossRef - Ala, A.A.; Olotu, B.B.; Ohia, C.M.D. Arch Basic Appl Med., 2018, 6(1), 61–65.
- Vajrabhaya,L.; Korsuwannawong, S. Journal of Analytical Science and Technology, 2018 9(1).
CrossRef - Thakkar, K. N.; Prasad, A .K.; Nayak, J.; Iyer, S.V.; Kumar, The Journal of Phytopharmacology, 2014, 3(6), 395-399.
- Shukla,A.; Vats, S.; Shukla, R. K. Indian J Pharm Sci., 2015, 77(5), 640–644.
CrossRef - Astadi, I.R.; Astuti, M.; Santoso, U.; Nugraheni, P.S. Food chemistry, 2009,112(3), 659-663.
CrossRef - Nakajima, J. I.; Tanaka, I.; Seo, S.; Yamazaki, M.; Saito, K. Journal of biomedicine & biotechnology, 2004, 2004(5), 241–247.
CrossRef - Wang, B.C.; Rui, H.; Chi M.L. Food technology and biotechnology, 2010, 48(1), 42-47.
- http://www.terraforma.ae/shop/product/pseuderanthemum-reticulatumgreen- eranthemum-green/
- Talreja, T.; Sirohi, P.; Sharma, T. International Journal of Pharmacy and Pharmaceutical Sciences, 2015, 7(4),416-418.
- https://www.iitg.ac.in/biotech/MTechLabProtocols/Carbohydrate%20estimation%20by%20Anthrone.pdf
- Plummer, D.T. An Introduction to Practical Biochemistry, Third Edition, Tata McGraw-Hill Education Pvt. Ltd. 2017.
CrossRef - Madhu, C.; Krishna, K.M.; Reddy, K.R.; Lakshmi, P.J.; Kelari, E.K. Int J Pharma Res Health Sci., 2017, 5(3), 1703-1706.
- Anschau, A.; Caruso, C. S.; Kuhn, R.C.; Franco, T.T. Brazilian Journal of Chemical Engineering, 2017, 34(1), 19 – 27.
CrossRef - Kripasana, K.; Xavier, J. Plant Sci. Today, 2020, 7(2), 264-274.
CrossRef - Delpech, R. J. Biol. Educ., 2000, 34, 206-210.
CrossRef - Idris, O.A.; Wintola, O.A.; Afolayan. Plants (Basel), 2019, 8(3):51.
CrossRef - Aslantürk, O.S. IntechOpen, 2017, Chapter 1, 1-53.
- Martín, J.; Kuskoski, E.M.; Navas, M.J.; Asuero, A.G. IntechOpen, 2017, Chapter 11, 205-255.

This work is licensed under a Creative Commons Attribution 4.0 International License.










