Microwave-irradiated synthesis and Biological applications of Transition metal (II) complexes of Unsymmetrical Quadridentate (ON NO donor) Schiff base ligand
K P Srivastava*, U S Yadav and Pragya Singh
Department of Chemistry, Jai Prakash University, Chapra-841301 (Bihar) India.
DOI : http://dx.doi.org/10.13005/ojc/370426
Article Received on : 10-Jul-2021
Article Accepted on :
Article Published : 13 Aug 2021
Reviewed by: Dr.Tasneem Mohammed
Second Review by: Dr. Likaa Khalid
Final Approval by: Dr. Saksit Chanthai
An efficient and environmentally benign method using microwave irradiation (MWI) have been applied for the synthesis of mononuclear square planar transition metal (II) [e.g. Co2+, Ni2+ and Cu2+] complexes with an unsymmetrical tetradentate bi-anionic Schiff base ligand. All the eco-friendly synthesized transition metal complexes were characterised by elemental analysis, conductivity & magnetic moment measurements and common spectroscopic methods (vis. IR, UV-Visible and 1H NMR spectroscopy respectively). The biological evaluation as anti-bacterial and human pathogenic anti-fungal activities of the investigating compounds show that the investigated complexes were found to be far active than the ligand towards tested microbes under the identical experimental conditions and the antimicrobial activity trend follows the order: Cu-complex > Co-complex > Ni-complex > Ligand
KEYWORDS:Anti-Microbial Activity; Microwave-Assisted Reaction; Square-Planar Geometry; Schiff Base; Transition Metals
Download this article as:| Copy the following to cite this article: Srivastava K P, U S Yadav, Singh P. Microwave-irradiated synthesis and Biological applications of Transition metal (II) complexes of Unsymmetrical Quadridentate (ON NO donor) Schiff base ligand. Orient J Chem 2021;37(4). |
| Copy the following to cite this URL: Srivastava K P, U S Yadav, Singh P. Microwave-irradiated synthesis and Biological applications of Transition metal (II) complexes of Unsymmetrical Quadridentate (ON NO donor) Schiff base ligand. Orient J Chem 2021;37(4). Available from: https://bit.ly/3sfpMTu |
Introduction
All over the world, the chemists are fascinated and inspired by the chemistry of transition metal complexes of the organic chelating ligands due to their academic, commercial and biochemical applications. This leads in the emergence of applied fields like coordination chemistry, organometallic chemistry, bioinorganic chemistry, inorganic medicinal chemistry and catalysis. Among the chelating organic ligands, the Schiff bases having azomethine group (>C=N-) have attracted chemists due to their easy synthetic approaches and complexation1-4. The literature survey revealed that the Schiff bases don’t only have the very good complexation capacity towards transition metal ions but also have innumerable applications in the various fields by varying denticity and basicity. The present trend in the chemical research on Schiff base coordination compounds has gyrated towards elucidation of their antimicrobial and other therapeutic activities5-8.
The Schiff bases or azomethines are the versatile class of organic compounds having fantastic and interesting biological activities. Therapeutically, these compounds and their metal complexes have a wide scope of biological activities, i.e., antibacterial, antifungal, antiviral, antimycobacterial, anti-malarial, anti-inflammatory, anti-oxidant, pesticidal, cytotoxic, enzyme-inhibitory and anti-cancer including DNA damage. Consequent upon the study of numerous applications of the Schiff bases in various fields of chemical sciences, the chemists have greater interest in developing efficient methods for the synthesis of them and their metal complexes. The microwave-irradiated reactions are the facile, efficient and eco-friendly approach for the synthesis of various Schiff base ligands and their transition metal complexes9-10.
The literature survey revealed that the synthetic chemists have been working from about three decades for the betterment of human living standard by reducing the chemical pollution during chemical syntheses. Now, most of the chemical syntheses have gone in the direction to design the processes that would be less harmful to human health and the environment through using eco-friendly methodologies of chemical synthesis. Among several environmentally benign methods of chemical synthesis, the microwave-promoted syntheses are the very significant one due to concise, efficient, simple procedure, easy work up, short reaction time, mild conditions and appreciable yields of the products 11-12.
As a part of our continuing effort13-17, we now report the evaluation of antimicrobial activity and structural elucidation of the microwave-promoted synthesized transition metal (II) complexes using unsymmetrical bi-anionic tetradentate (N2O2 donor) Schiff base ligand under environmental benign conditions in the present research article. This article describes the extensive analysis of the synthesized tetradentate (ON NO donor) ligand and its bivalent Co, Ni and Cu complexes via conventional thermal method and microwave assisted method and their biological evaluation as anti-bacterial and human pathogenic anti-fungal activities. Although, single crystals of the investigated M(II) complexes have not been isolated; but their analytical, magnetic and conductance data and spectroscopic studies enabled us to propose the conceivable geometry of undertaken complexes. Further, it presents the antimicrobial activities of the investigated ligand and its metal complexes against gram- positive bacteria, gram-negative bacteria and human pathogenic fungi.
Experimental Study
Materials and Methods
All the reagents and solvents were of analytical/spectroscopic grade. These chemicals were purchased from Aldrich-Sigma Ltd. These chemicals were used without advance purification during the synthesis.
An Electro-thermal 9100 apparatus was used to determine the uncorrected melting points of the investigated compounds. The conductivity measurements of the investigated compounds were performed in DMF by bridge model PW 9501 having Philips PW 9515/10 conductivity cell. Gouy’s method was used to determine the magnetic susceptibility of the investigated complexes using Hg[Co(NCS)4] (µg =16.44×10-6) as a standard or reference. A Carlo-Ebra EA1110 CHNO-S analyser was used for the elemental analyses of the investigated compounds. A Shimadzu (Model UV-1900i) UV-Visible spectrophotometer was used for the record of electronic spectra of the investigated compounds. The infrared spectra of all the investigated compounds were obtained as KBr discs in the range 200-4000 cm-1 on a Shimadzu Infrared spectrophotometer (Model IR Affinity-IS). The 1H NMR spectra of all the undertaken compounds were obtained on a Varian NMR model A-60 spectrometer using CDCl3 solvent and DMSO-d6 was used as an internal reference (TMS=0 ppm).
The microwave promoted synthetic reactions were carried out in a converted usual microwave oven (model 2001 ETB, Bajaj Electrical Ltd.) fitted with rotating tray and a power source 230 V, at an output energy of 800 W and 2450 MHz frequency. The on/off cycling method was used to control the temperature of the reactions. The thin layer chromatography (TLC) on pre-coated silica gel GF254 plates (E-Merck) was used to monitor the progress of the microwave promoted reactions and the purity of the products was determined using appropriate solvent systems.
The anti-microbial activities of the tetradentate ligand and its investigated complexes were studied by the common disc diffusion method. The nutrient agar medium and spread dextrose agar medium were used for anti-bacterial activity and anti-fungal activity respectively in disc diffusion method.
Microwave-assisted synthesis of Ligand
The undertaken tetradentate ligand [N-(acetophenylidene)-N-(salicylidine)-ortho-phenylenediamine {H2(aceph-sal)opd}] was obtained by condensation of a 1:1:1 molar mixture of o-hydroxy acetophenone, salicydehyde and o-phenylenediamine in aqueous ethanolic medium (green solvent) at ambient temperature for about six minutes in the microwave oven and then cooled till yellowish solid. The yellowish solid product was washed out with ethanol, then recrystallized with acetone and finally dried under reduced pressure over anhydrous CaCl2 in a desiccator (scheme-1). In comparison to the common thermal method, the green synthetic approach requires short time and produced better yield (about 77%) of the unsymmetrical new ligand. The completion time and the yield of the product using conventional method and green chemical approach are presented in table-1 for comparison.
Microwave assisted synthesis of Metal Complexes
The undertaken transition metal (II) complexes were obtained by refluxing an aqueous-ethanol solution of 1:1 (metal:ligand) stoichiometric ratio of metal acetate and unsymmetrical tetradentate Schiff base in the microwave oven adding a few drops of triethyl amine as a catalyst. The microwave-assisted synthetic process was completed in a short time (5-12 minutes) and produced better yield (56-70%) of the metal complexes (scheme-1) in comparison to the common conventional method. The resultant coloured solid products were filtered and recrystallized from DMF, washed out with ethanol and then dried under reduced pressure over anhydrous CaCl2 in a desiccator.
 |
Scheme 1: Microwave assisted synthesis of unsymmetrical tetradentate ON NO donor ligand and its M(II)-complexes (where, M = Co2+, Ni2+& Cu2+) MWI = Microwave Irradiation. |
Antimicrobial Activity
The disc diffusion method18 was used for the study of anti-bacterial activity and anti-fungal activity of the investigated ligand and its transition metal (II) complexes. The in vitro anti-bacterial activity of investigated compounds was performed against two gram-positive bacteria [Staphylococcus aureus (SA) and Enterococcus faecalis (EF)], two gram-negative bacteria [Escherichia coli (EC) &Staphylococcus mutans (SM)] using chloramphenicol as standard reference but the anti-fungal activity was performed against fungal strains [Candida albicans (CA) &Aspergillus niger (AN)] using griseofulvin as standard reference of the same concentration under identical conditions.
The investigated ligand and its transition metal complexes were dissolved in DMSO (without inhibitory activity) to appropriate concentration. The sterile disks were drenched in undertaken test compounds and were carefully placed on incubated agar surface. The petri- dishes were incubated for 24 hours at 643K for bacteria and for 48 hours at 643K for fungi. Finally, the zone of inhibition was carefully measured. Each test was performed in triplicate in individual experiment and the mean is reported in table-4.
Results and Discussion
All the investigated transition metal (II) complexes were stable and non-hygroscopic coloured solid at RTP. These complexes have range of melting points and these decompose on heating at higher temperature than respective melting point range. These complexes were generally insoluble in common natural organic solvents at room temperature.
The spectroscopic and micro-analytical studies of investigated complexes revealed that their composition corresponds to [ML] stoichiometry and mononuclear nature. Their determined molar conductance values (4.5 to 18 ohm-1 cm2 mol-1) were too less to describe for any dissociation of the investigated metal (II) complexes in DMF at room temperature which revealed their non-electrolytic nature19. The analytical data of the undertaken compounds with their physical properties are presented in table-1. Further, the comparisons of results of conventional and green chemical procedures related to undertaken compounds are also shown in table-1.
The experimental results revealed that the green synthetic reactions were completed in relatively shorter period with better yields compared to the conventional thermal procedure.
Table 1:Comparison of conventional method and microwave-assisted method, micro-analytical and physical data of the compounds under investigation
|
Compounds (colour) |
Reaction Period CM(hrs.) MM(min.) |
% Yield CM (MM) |
Mol. Weight |
Melting point (in K) |
Elemental Analysis % Calculated (Found) |
Conductance (Ohm-1 cm2 mol-1) |
|||
|
C |
H |
N |
M |
||||||
|
C21H18N2O2 (Pale yellow) |
2 hrs. (6 min.) |
60 (77) |
344 |
512 |
76.74 (76.70) |
5.81 (5.80) |
8.13 (8.10) |
— |
20 |
|
[Co(C21H16N2O2)] (Blue) |
2.5 hrs. (6 min.) |
47 (70) |
400.93 |
537 |
65.84 (65.80) |
4.48 (4.50) |
6.98 (6.90) |
14.67 (14.70) |
18 |
|
[Ni(C21H16N2O2)] (Red) |
2.5 hrs. (8 min.) |
44 (68) |
400.71 |
557 |
65.88 (65.90) |
4.49 (4.50) |
6.98 (7.00) |
14.65 (14.60) |
12 |
|
[Cu(C21H16N2O2)] (Pale brown) |
2.5 hrs. (10 min.) |
42 (65) |
405.54 |
596 |
65.09 (65.10) |
4.43 (4.45) |
6.90 (7.00) |
15.67 (15.70) |
4.5 |
CM = Conventional Method; MM = Microwave assisted Method
Infrared Spectral Studies
The IR spectral data of the investigated microwave-irradiated synthesized transition metal (II) complexes are presented in table-2 which contained all the absorption bands of ligand except specific bands and some new absorption bands which revealed the coordination of the chelating ligand to the central M(II) ion through N and O donors. The tentative assignments of the observed infrared spectra of the investigated complexes were compared with that of the chelating ligand to determine the participation of ligation sites (N and O) in the complexes.
The nonappearance of the unlinked N-H and non-coordinated -OH group20 in the IR spectra of the investigated ligand and the complexes was indicated by the absence of bands between 3100 and 4000 cm-1. Further, due to the unsymmetric nature of the chelating free ligand and its investigated complexes, two bands were observed for each of the following bands taking their lineage from the aldehyde and ketone:

The disappearance of the absorption band due to amino group (-NH2) in the IR spectra of the free ligand indicated the condensation of the amino groups of diamines with the aldehyde /ketone or both. The IR absorption band of azomethine group (>C=N-) in the free ligand was shifted from 1560-1650 cm-1 to lower frequency at 1555-1635 cm-1 in the investigated M(II) complexes revealed the coordination of azomethine N to the central M(II) ion21.
Further, the shifting of phenoxide band [ν(C-O) band] from 1257-1335 cm-1) of the free ligand to 1280-1366 cm-1 in the investigated complexes indicated protonation and ligation of the phenolic oxygen to the central M (II) ion in the investigated complexes22.
Table 2: Observed infrared spectral bands (cm-1) of investigated M (II)-complexes
|
Complexes |
ν(C=N) |
ν(C-O) |
ν(M-N) |
ν(M-O) |
|
[Co(C21H16N2O2)] |
1635, 1575 |
1360, 1282 |
455, 510 |
580, 425 |
|
[Ni(C21H16N2O2)] |
1632, 1586 |
1365, 1280 |
457, 508 |
585, 429 |
|
[Cu(C21H16N2O2)] |
1630, 1590 |
1366, 1285 |
458, 505 |
575, 421 |
Thus, the infrared spectral studies revealed that the investigated Schiff base ligand acted as bi-anionic tetradentate (designated as ON NO donor) ligand since it coordinated with metal (II) ion via azomethine N and the phenolic O leading to the appearance of the new bands at 455-510 cm-1 and 421-585 cm-1 which assigned to the M-N and M-O vibrations respectively which confirming the participation of N & O atoms in the coordination with the central metal (II) ions in the investigated complexes. These IR absorption bands were absent in the spectra of the free Schiff base ligand.
Electronic Spectral Studies & Magnetic Properties
The electronic or UV-Visible spectral studies are quite helpful in the elucidation of the structure of the compounds in association with other spectroscopic methods. The stereochemistry or geometry of the coordination compounds can be assigned on the basis of position and number of d-d transition peaks in their electronic absorption spectra.
For the assignment of the plausible structure around the M(II) ion in the investigated complexes, the electronic spectra of free ligand and its transition metal complexes were recorded in 10-3 M of each in DMSO in the range of 200-1100 nm at room temperature. The obtained d-d transitions and charge transfer transitions are presented here in table-3.
On the basis of the electronic spectral studies of the undertaken complexes, either a square planar or a tetrahedral configuration may be possible for them but their observed magnetic moment values clarified the square planar geometry of all the investigated complexes.
Table 3: Observed electronic absorption bands, magnetic moments and stereochemistry of M(II)-complexes.
|
Complex |
d-d transition / cm-1 (ε / cm-1 mol-1) |
CT/ cm-1 (ε / cm-1 mol-1) |
μeff (BM) |
Stereochemistry |
|
[Co(C21H16N2O2)] |
18928 (82), 22518 (201), 24872 (273) |
29576 (195) |
2.39 |
Square planar |
|
[Ni(C21H16N2O2)] |
20780 (152), 25770 (315) |
30575 (182) |
0.00 |
Square planar |
|
[Cu(C21H16N2O2)] |
23285 (205) |
29320 (197) |
1.81 |
Square planar |
The three electronic absorption bands in the electronic solution spectra of Co(II) complex distinctly revealed its low-spin square-planar or distorted square-planar geometry23, which is substantiated by the determined magnetic moment (2.39 BM) corresponding to single electron with an ostensibly greater orbital contribution in Co2+ ion in the complex. The observed other intense band was assigned as intra-ligand or charge-transfer transition.
The observed two bands corresponding to 1A1g → 1B1g and 1A1g → 1Eg transitions in the electronic spectra of the investigated Ni(II) complex revealed its square planar geometry24 and one intense band was assigned as intra-ligand or charge-transfer transition. The observed zero BM effective magnetic moment value of Ni(II) complex supported its square-planar geometry.
The electronic solution spectra of Cu(II) complex consisted A broad unsymmetrical band assigned to 2B1g → 2A1g transition in the electronic solution spectra of undertaken Cu(II) complex disclosed its four-coordinated square-planar geometry25-27. The other intense band was due to charge-transfer or intra-ligand transition. The assigned square-planar geometry of the complex was supported by the determined 1.81 BM effective magnetic moment. This observed effective magnetic moment value may be assigned as the outcome of the contribution of orbital and spin-orbit coupling.
1H NMR Spectral Studies
The unsymmetrical nature of the undertaken chelating ligand was confirmed by the visual aspect of two different peaks; one for azomethine protons and second for the phenolic protons. The higher of the Two signals for both the azomethine and the phenolic protons was assigned to the azomethine/phenolic proton linked to the acetophenone ring have values, but the lower values two signals for both the azomethine/phenolic protons was assigned to the azomethine/phenolic proton linked to the salicyldehyde portion of the ligand.
The observed signals between 8.2 and 9.0 ppm were assigned to the methine protons of the azomethine group but the multiple signals in the range 6.8 – 8.2 ppm were ascribed to chemical shifts for aromatic protons. The downfall shifting of a singlet in the region 12.0-15.5 ppm of O-H protons of the phenolic group were attributed to intra-molecular hydrogen bonding. A triplet signal at 1.8 ppm assigned to the methyl protons of the acetophenone and a singlet appeared at 1.3 ppm is assigned for aldehydic proton of salicyldehyde28.
The deshielding effect of electron withdrawing group like acetophenone and the H-bonding between the phenolic proton and the azomethine nitrogen were responsible for the further downfall and observed difference in the signals of the protons29.
Proposed Geometry of M(II) Complexes
The square-planar geometry (figure-1) is being provisionally proposed for the undertaken M(II) complexes on the basis of the observations obtained from the spectral and magnetic properties. Evidently, it is found that the ligand appears to be a bi-anionic tetradentate (ONNO) donor and the elemental analysis data and molecular weight determination show the mononuclear nature of the investigated M(II) complexes.
 |
Figure-1: Proposed square-planar geometry of investigated M(II) complexes Click here to View figure |
Studies of Antimicrobial Activities
The experimentally studied results related to the different anti-microbial actions of the undertaken free ligand and the M(II) complexes are presented in table-4. On the basis of the values of minimum inhibitory concentration (MIC) of the free ligand and its M(II) complexes it is found that all the complexes have greater activity than the ligand which may be probably due to their lipophilic nature and coordination effect of ligand to central metal M(II) ions, that can be explained by the chelation theory [30]. The observed trend of antimicrobial action follows the order:
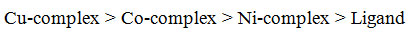
Table 4: The in vitro antimicrobial activity [MIC in µg/mL] of the ligand and its M(II) complexes.
|
Compounds |
MIC {µg / mL} |
|||||
|
Bacteria |
Fungi |
|||||
|
Gram-positive |
Gram-negative |
|||||
|
SA |
EF |
EC |
SM |
CA |
AN |
|
|
C21H18N2O2 |
13 |
11 |
13 |
12 |
19 |
20 |
|
[Co(C21H16N2O2)] |
6 |
5 |
7 |
4 |
9 |
8 |
|
[Ni(C21H16N2O2)] |
11 |
9 |
11 |
8 |
15 |
16 |
|
[Cu(C21H16N2O2)] |
8 |
7 |
9 |
6 |
12 |
11 |
|
Chloramphenicol |
0.25 |
1.5 |
2.3 |
1.7 |
3.2 |
3.5 |
|
Griseofulvin |
– |
– |
– |
– |
8 |
11 |
Conclusions
A more adequate and facile synthesis of a new pharmaco-active ligand in single step by refluxing o-hydroxy acetophenone and salicyldehyde with o-phenylenediamine in aqueous-ethanol as a green solvent and its bivalent transition metal complexes bearing possible pharmacophore were obtained under microwave irradiation. The high yields of the compounds within less time under MWI were obtained in comparison to conventional method. The prepared ligand {H2(aceph-sal)opd} coordinated to the M(II) ion in a bi-anionic tetradentate (ONNO donor) mode. The square-planar geometry of the undertaken mononuclear M(II) complexes has been proposed on the basis of elemental analysis, molar conductance, magnetic moment measurement, IR, UV-Visible and 1H NMR spectral observations. The anti-microbial activities indicated that the investigated M(II) complexes possess comparatively stronger anti-bacterial and anti-fungal activities than the ligand and the antimicrobial activity trend of the investigated compounds follows the order:

Acknowledgement
One of the authors (Dr. Pragya Singh) expresses her thanks to late Prof. S. N. Vidyarthi, Ex-Dean, Faculty of Science, J.P. University, Chapra, Bihar (INDIA) for his keen interest and critical suggestions during her research work.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- Comba P.Coord. Chem. Rev., 123:1-48, (1993)
CrossRef - Yamada S. Coord. Chem. Rev., 190-192:535-555, (1999)
CrossRef - Cozzi P.G. Chem. Soc. Rev., 33:410-421,(2004)
CrossRef - Vigato P. A. and Tamburini S. Coord. Chem. Rev., 248:1717-2128, (2004)
CrossRef - Ben-saber S.M.; Maihub A.A.; Hudere S.S. and El-ajaily M.M. Microchemical J., 81:91-194, (2005)
CrossRef - Radecka-Paryzek W.; Patroniak V. and Lisowski J. Coord. Chem. Rev., 249:2156-2175, (2005)
CrossRef - Chohan Z.H.; Arif, M.; Akhtar, M.A.and Supuran, C.T. Bioinorganic Chemistry and Applications, 1-11,(2006)
CrossRef - Gupta, K. C.and Sutar A. K. Coord. Chem. Rev., 52 (12-14):1420-1450, (2008)
CrossRef - Polshettiwar, V. Aqueous Microwave Assisted Chemistry: Synthesis and Catalysis, RSC, Cambridge, UK; (2010)
CrossRef - Rao V. K.; Reddy S. S.; Satheesh K. B.; Mohan K. R.; Naidu K.R.M.and Raju C.N. Green Chemistry Letters and Reviews, 3:3, 217-223,(2010)
CrossRef - Leadbeater, N.E.; Microwave Heating as a tool for Sustainable Chemistry, 2010, CRC Press, Boca Raton, Florida, USA, (2010)
CrossRef - Sheldon, R. A.; “Matrices of Green Chemistry and Sustainability: Past, Present and Future”, ACS Sustainable Chem. Eng., 6:32-48, (2018)
CrossRef - Srivastava K. P.; Anuradha Singh and Suresh Kumar Singh IOSR Journal of Applied Chemistry,7(4): 16-23, (2014)
CrossRef - Srivastava K.P.; Sunil Kumar Singhand Bir Prakash Mishra der Pharma Chemica, 2015,7 (1):121-127, (2015)
CrossRef - Srivastava K. P.; Putul O. P.and Nagendra Kumar Der Pharma Chemica,8(3):105-116, (2016)
- Srivastava K. P.and Anuradha Singh Journal of Applicable Chemistry,6(4):599-606, (2017)
- Srivastava K. P.; Pragya Singh and Yadav U.S. IOSR Journal of Applied Chemistry,13(9): 52-58, (2020)
- Greenwood D.; Snack R. and Peurtherer J. Medical microbiology: A guide to microbial infections: Pathogenesis, immunity, laboratory diagnosis and control, 15th edn., (1997)
- Geary W. J. Coord. Chem. Rev., 7 (1):81-122.(1971)
CrossRef - Nakamoto K. Infrared spectra of Inorganic and Coordination Compounds; 2nd edn. Wiley-Intersciences, New York(1970)
- Bellamy L. J. The Infrared Spectra of complex molecules; 3rd edn. Chapman and Hall, London, Vol. 233, (1975)
CrossRef - Adams D. M. Metal-ligand and related vibrations, Edward Arnold, London, 248- 284, (1967)
CrossRef - Manhas B.S.; Verma B.C.and Kalia S.B. Polyhedron, 14 (23-24):549-3556, (1995)
CrossRef - Boghaei D.M.and Lashanizadegan M. J. Sci. I. R. Iran, 11(4):301-304, (2000)
- Bu, X.R.; Jackson, C.R.; Derveer, D.V.; You, X.Z.; Meng, Q.J. and Wang, R.X. Polyhedron, 16(17):2991-3001, (1997)
CrossRef - Satyanarayana D. N.; Electronic absorption spectroscopy and Related Techniques, Universities Press Ltd., India, (2001)
- Mabbs F.E.and Machin D.J. Magnetism and Transition Metal Complexes, Chapman and Hall, London, (1973)
- Aranha P.E.; Do SantoM.P.; Romera S.and DockalE.R. Polyhedron, 26: 1373-1382, (2007)
CrossRef - Percy G.C.and Thornton D.A.; J.Inorg. Nucl. Chem.,34 (11):3357-3367, (1972).
- Tweedy B. G.; Phytopatholo., 55: 910-919, (1964)

This work is licensed under a Creative Commons Attribution 4.0 International License.









