Reactions of MoO2Cl2 and MoOCl4 with 2-Mercaptopyridine, 4-Phenylimidazole-2-thiol and 6-Mercaptopurine Monohydrate
Deepika Rani1 , Gursharan Singh2*
, Gursharan Singh2* and Seema Sharma2
and Seema Sharma2
1Punjab Technical University, Kapurthala, India.
2Department of Applied Chemistry, Giani Zail Singh Campus College of Engineering and Technology, Dabwali Road, MRSPTU Bathinda-151001-India.
Corresponding Author E-mail: gursharans82@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370105
Article Received on : 18-Jan-2021
Article Accepted on :
Article Published : 09 Feb 2021
MoO2Cl2/MoOCl4 have been reacted with 4-phenylimidazole-2-thiol/6-mercaptopurine monohydrate/2-mercaptopyridine in acetonitrile solvent in unimolar/bimolar proportions at room temperature. The products thus obtained are: MoOCl3(C9H8N2S), [1]; Mo2O3Cl6(C9H7N2S)(CH3CN)2, [2]; Mo2O3Cl8(C9H7N2S)2(CH3CN)2, [3] and Mo2O4Cl4(C5H4NS-SN4C5), [4]. These products were studied by various techniques: infrared, proton NMR, liquid/gas chromatography-mass spectrometry, elemental analyses. Owing to the sensitivity of the products to air and moisture, the reactions and work ups were performed in vacuum line purged with oxygen by flushing dry nitrogen in it. Ions observed in mass spectrum are concurrent with the depicted formulae.
KEYWORDS:Acetonitrile solvent; Infrared; liquid/gas chromatography-mass spectrometry; MoO2Cl2; MoOCl4; 2-mercaptopyridine; 6-mercaptopurine monohydrate; 4-phenylimidazole-2-thiol; Proton NMR, DMSO-d6
Download this article as:| Copy the following to cite this article: Rani D, Singh G, Sharma S. Reactions of MoO2Cl2 and MoOCl4 with 2-Mercaptopyridine, 4-Phenylimidazole-2-thiol and 6-Mercaptopurine Monohydrate. Orient J Chem 2021;37(1). |
| Copy the following to cite this URL: Rani D, Singh G, Sharma S. Reactions of MoO2Cl2 and MoOCl4 with 2-Mercaptopyridine, 4-Phenylimidazole-2-thiol and 6-Mercaptopurine Monohydrate. Orient J Chem 2021;37(1). Available from: https://bit.ly/3aMjcf8 |
Introduction
6-Mercaptopurine ring system may be considered as if a pyrimidine ring has been fused to an imidazole ring. Electrons of 6-mercaptopurine are highly delocalized. The ring is susceptible to both electrophilic and nucleophilic attacks. 6-Mercaptopurine1,2 is used as chemotherapy drugs for treatment of autoimmune diseases and cancer like leukemia, ulcerative colitis and Crohn’s disease. Mercaptopurine is sold as purinethol. It is a class of medication known as purine antagonists and works by stopping the growth of cancer cells. Many transition metal complexes of 6-mercaptopurine are reported3,4. Some of transition metal complexes of 6-mercaptopurine have higher anticancer activity than that of 6-mercaptopurine5,8. Divalent transition metals coordinate5,7,9 through S and N atoms of 6-mercaptopurine
Heterocyclic thioamides like 4-phenylimidazole-2-thiol having N and S-donor ligands are biologically active and are used as anti-thyroidal agents10. Imidazothiazole structural unit containing heterocyclic compounds are biologically active11. Many enzymes and receptors12,15 can be inhibited by them. They are used in diuretic16, fungicidal17, antihelmintic18, antitumor19,24, antidiabetic25 and antimicrobial26,27 drugs.
There are many biochemical applications28-30 of metal complexes with thioligands.
Aim of Investigation
MoO2Cl2 and MoOCl4 are known to react with a variety of ligands. The author earlier investigated31,37 reactions of MoO2Cl2 with various diaminoalkanes, alkanediols, amides, imides, thiols and aromatic azoles.
The author earlier also investigated31-33, 38-39 reactions of MoOCl4 with various diaminoalkanes, amides, imides, alkylpyridines, mercaptopyridine, mercaptopyridine-N-oxide sodium, 2-thiazoline-2-thiol, alkylpyrrolidine, alkylpiperidine and aromatic azoles.
The author has reported earlier also molybdenum compounds containing 4-phenylimidazole-2-thiol, 6-mercaptopurine monohydrate and 2-mercaptopyridine.
In view of the wide applications of the transition metal complexes, now author has prepared molybdenum complexes of 2-Mercaptopyridine, 4-Phenylimidazole-2-thiol and 6-Mercaptopurine monohydrate on reaction with MoO2Cl2 and MoOCl4. The complexes have been characterised by elemental analysis, Mass, IR and NMR techniques. All preparations and work ups have been done under rigorous moisture/air free environment.
Materials and Methods
MoO2Cl2, MoOCl4, 4-phenylimidazole-2-thiol, 6-mercaptopurine monohydrate and 2- mercaptopyridine used were manufactured by Sigma-Aldrich. We used them without any further treatment. Owing to the sensitivity of the products to air and moisture, the reactions and work
ups were performed in vacuum line purged with oxygen by flushing dry nitrogen in it. The reactions were carried out for 6-8 hours with continuous stirring using pressure stabilised dropping funnel. The products were filtered through filtration unit fitted with G-4 crucible and isolated.
Molybdenum was determined by oxinate40 gravimetric method. Chlorine was determined by silver chloride40 gravimetric method. Thermo Finnigan Elemental Analyser was used to determine other elements. Perkin-Elmer 400 FTIR Spectrometer, in the range 4000 – 400 cm-1 was used to obtain spectra using KBr disks. 1H-NMR spectra were recorded in solvent DMSO-d6
using Brucker Avance-II 400 NMR. Liquid Chromatography-Mass spectra were obtained in the range 0 – 1100 m/z. These facilities were provided by Panjab University, Chandigarh (India).
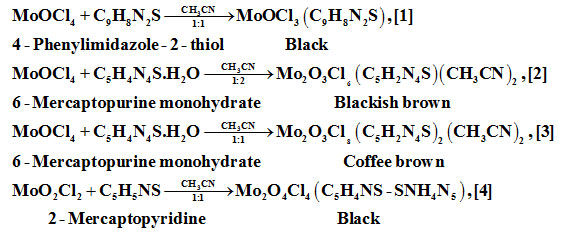
Preparation of Compounds [1]-[4]
Disproportionation/rearrangement might have occurred during the course of reactions. The source of the products is indicated below the products.
Results and Discussions
Analytical Measurements
Estimation of elements (percentage) are given in Table-1. Theoretical values are given in parenthesis.
Table 1: Analytical Measurements.
|
Products |
Mo |
Cl |
C |
H |
N |
S |
|
MoOCl3(C9H8N2S), [1] (Black/394.5) |
23.66 (24.33) |
26.78 (27.00) |
28.13 (27.38) |
02.78 (02.03) |
6.13 (7.10) |
7.23 (8.11) |
|
Mo2O3Cl6(C9H7N2S)(CH3CN)2, [2] (Blackish brown/685.0) |
27.53 (28.02) |
30.13 (31.09) |
14.95 (15.76) |
1.65 (1.16) |
12.78 (12.26) |
4.54 (4.67) |
|
Mo2O3Cl8(C9H7N2S)2(CH3CN)2, [3] (Coffee brown/906.0) |
20.78 (21.19) |
30.73 (31.34) |
17.66 (18.54) |
1.17 (1.10) |
15.14 (15.45) |
3.23 (3.53) |
|
Mo2O4Cl4(C5H4NS-SN4C5), [4] (Black/618.0) |
31.67 (31.06) |
22.60 (22.97) |
18.54 (19.41) |
1.48 (1.29) |
4.34 (4.53) |
09.55 (10.35) |
FTIR Spectra
Absorption at 3137 cm-1 in1 refers to N-H stretching of 4-phenylimidazole-2-thiol37,41-43 (Table-2). There is no υ(S-H) in the range 2551-2602 cm-1 of1 indicating S-H group is not present it. Peaks at 1261 cm-1 and 1106 cm-1 in [1] correspond to C=S stretching. υ(C=O) is higher than υ(C=S), because carbonyl bond is stronger and more polar than thiocarbonyl bond. Carbonyl bond absorptions are more intense than that of thiocarbonyl bond. C-S stretching is detected at 761 cm-1. C-S stretching observed points out to the existence of Mo-S bonds. υ(Mo-S)9,49 appears at 498 cm-1. Terminal Mo=O group absorbs44 in the span 991 cm-1-1008 cm-1. Mo=O stretching45-47 was observed at 986 cm-1. There is thiol-thione tautomerism in imidazole-2-thiones37,43. Mo=O stretching shows downward shift owing to S→Mo coordination37, 48 of 4-phenylimidazole-2-thiol. Ligand coordination is trans to Mo=O. It implies that 4-phenylimidazole-2-thione reacts in thiol form. This fact is further evident by the higher value of υ(C=N).
Table 2: FTIR Spectra
|
Mode |
(4-Phenylimidazole-2-thiol)37,41-43 |
[1] |
|
υ(N-H) |
3129, 3248 s |
3137.28 v s |
|
υ(S-H) |
– |
– |
|
υ(C=N), υ(C=C) |
1560, 1502, 1463 |
1621.30 s, 1597.31 s, 1505.38 m, 1457.37 m |
|
υ(C=S) |
1261, 1109 |
1261 m, 1106 m |
|
υ(C-S) |
780 |
761.27 v s |
|
υ(Mo-S)9, 49 |
– |
498.44 v w |
|
Terminal υ(Mo=O)45-47 |
– |
986.30 v s |
Absorptions at 3400 cm-1 in [2] and 3407 cm-1 in [3] suggest υ(N-H) of pyrimidine ring of 6-mercaptopurine9,50, 58. υ(N-H) of imidazole ring are missing due to absence of N-H bond of imidazole ring (Table-3). This shows that there is Mo-N bond formation. No υ(S-H) peak has been observed in [2] and [3]. This means that 6-mercaptopurine has participated in thiol form.
This is further supported by higher C=N stretching in these complexes. Lower N-H stretching implies coordination50 of 6-mercaptopurine to molybdenum. υ(Mo-S)9,49 appears at 729 cm-1 and 728 cm-1 in [2] and [3], respectively. υ(Mo=O) absorptions at 973 cm-1 in [2] and 970 cm-1 [3], reveal terminal (Mo=O)45-47 in them.
Table 3: This shows that there is Mo-N bond formation.
|
Mode |
6-Mercaptopurine monohydrate9, 50, 58 |
[2] |
[3] |
|
υ(N-H) Imidazole |
3523 |
– |
– |
|
υ(N-H) Pyrimidine |
3376 |
3400.5 v s |
3407.1 v s |
|
υ(C-H) |
3095.0, 2993.8 |
– |
– |
|
υ(S-H) |
2671.5 |
– |
– |
|
υ(C=C) |
1669.7 |
– |
– |
|
δ(C=N) Imidazole |
1620 |
1626.8 v.s. |
1626.1 v.s. |
|
δ(C=N) Pyrimidine |
1393 |
1402.1 m |
1401.6 m |
|
υ(C-N) |
1343.8 |
1335.2 w |
1334.9 w |
|
δ(N-H) |
1526.6 |
1504.1 sh |
1504.6 sh |
|
υ(C=S) |
1193 |
1027.24 w |
1027.1 w |
|
υ(Mo-S)9,49 |
– |
729.1 m |
728.6 m |
|
υ(Mo-N)9 |
– |
496.2 w |
496.9 w |
|
Terminal υ(Mo=O)45-47 |
– |
973.1 |
970.4 s |
2-Mercaptopyridine33,51-55 shows υ(N-H) at 3177 cm-1 and υ(S-H) at 2708 cm-1 (Table-4). Bands at 3383 cm-1 shows that [4] has N-H group. υ(S-H) around 2708 cm-1 is missing pointing to S-H group absence in [4]. υ(Mo=O) peak at 983 cm-1 reveals presence of terminal Mo=O group45-47 [4].
Table 4: FTIR frequencies in cm−1
|
Mode |
(2-Mercaptopyridine)33,51-55 |
[4] |
|
υ(N-H) |
3177 |
3383 vs |
|
υ(C-H) |
3053, 2928, 2880 |
3128.2 s, 2889.1 m |
|
υ(S-H) |
2708 m |
– |
|
Ring breathing modes & Hydrogen in plane wagging |
1614 s, 1577 vs, 1503 s, 1447 s, 1419 s |
1604.1s, 1578.2 s, 1496.2 m |
|
υ(C=N) ring |
1274 m |
1273.2 w |
|
δ(C-H) in plane bending |
1247 m |
1227.4 w |
|
υ(C=S) |
1187 vs, 1144 vs |
1177.3 w |
|
δ(C-S), Hydrogen out of plane bending out of plane |
746, 614 |
767 vs, 707.3 w |
|
Terminal υ(Mo=O)45-47 |
– |
983.2 s |
1H NMR Spectra
Chemical shift of 4-phenylimidazole-2-thiol37,56-57 N-H occurs at 12.9 δ. alcoholic, phenolic, amino and thiolic protons have no specific chemical shift, because these are labile. Spectrum is generally recorded in a solvent, N-H chemical shift (Table-5) is missing in [1]. S-H in 4-phenylimidazole-2-thiol has chemical shift at 12.14 δ. S-H peak is missing in [1]. S-H group does not exist in [1]. [1] shows upfield chemical shift of ring protons and H-5.
Table 5: 1H NMR Spectra .
|
Protons |
(4-Phenylimidazole-2-thiol)37,56-57 |
[1] |
|
N-H |
12.99 |
– |
|
S-H |
12.14 |
– |
|
H-5 |
7.51 |
7.39 |
|
Aromatic H-2 and H-6 |
8.13 |
7.72 |
|
Aromatic H-3 and H-5 |
7.51 |
7.39 |
|
Aromatic H-4 |
7.41 |
– |
6-Mercaptopurine monohydrate58 aromatic N-H absorption shits to 7.8 δ, 7.15 δ in [2] and [3], respectively showing that this N-H is not involved in bonding (Table-6). S-H peak is missing in [2] and [3], indicating presence of Mo-S bond in them.
Table 6: 1H NMR absorptions in ppm
|
Protons |
6-Mercaptopurine monohydrate58 |
[2] |
[3] |
|
N-H (Arom) |
7.2 |
7.15 |
7.92 |
|
S-H |
1.2 |
– |
– |
|
N-H |
1.4 |
1.85 |
1.84 |
|
CH3CN |
– |
2.05 |
2.01 |
Comparison of 2-mercaptopyridine33,53 spectrum with that of [4] shows that absorptions move downfield (Table-7). There is no N-H peak. Deshielding is due to an increase in π electron density on coordination in the C–N bond.
Table 7: 1H NMR absorptions in ppm .
|
Protons |
(2-Mercaptopyridine)3,53 |
[4] |
|
N-H |
– |
– |
|
C3-H |
7.32 |
7.67 |
|
C4-H |
6.81 |
7.28 |
|
C5-H |
7.47 |
7.81 |
|
NC-H |
7.69 |
8.48 |
Mass Spectra (LC-MS)59
There is formation of [SOCl2]+ and [SOCl]+ on fragmentation60 of [4]. Ions observed in mass spectrum are concurrent with the depicted formulae (Tables-8, 9),
 |
Table 8: (Fragmentation) |
Table 9: (m/z values of some fragments).
|
Comp. |
Fragment |
Calculated52 |
Observed |
Relative abundance |
|
[1] |
[SOCl2]+ |
117.9 |
118.9 |
100% |
|
[SOCl]+ |
82.93 |
84.0 |
17% |
|
|
[2] |
[C5H4N4S]+ |
152.01 |
154.14 |
100% |
|
[C5H4N4S]2+ |
76.00 |
77.51 |
26% |
|
|
[MoOCl2]+ |
183.83 |
182.85 |
66% |
|
|
[Mo2OCl4(CH3CN)2]+ |
433.73 |
429. |
3% |
|
|
[Mo2O3Cl4C5H2N4S(CH3CN)2]+ |
615.72 |
610.19 |
8% |
|
|
[Mo2O3Cl6C5H2N4S(CH3CN)2]+ |
685.66 |
684.21 |
4% |
|
|
[3] |
[C5H4N4S]+ |
152.01 |
154.15 |
100% |
|
[MoOCl2]+ |
183.83 |
182.86 |
20% |
|
|
[Mo2OCl4(CH3CN)2]+ |
433.73 |
437.21 |
5% |
|
|
[Mo2O3Cl4C5H2N4S(CH3CN)2]+ |
615.72 |
610.19 |
6% |
|
|
[Mo2O3Cl6C5H2N4S(CH3CN)2]+ |
685.66 |
684.22 |
3% |
|
|
[Mo2O3Cl8(C5H2N4S)2(CH3CN)2]+ |
— |
— |
— |
|
|
[4] |
[C5H5NS]+ |
111.01 |
111.00 |
4% |
|
[C5H4NS-SN4C5]+ |
220.1 |
220.98 |
93% |
|
|
[C5H4N-SN4C5]+ |
188.04 |
189.01 |
100% |
|
|
[Mo2O3(C5H4NS-SN4C5)]+ |
463.80 |
460.04 |
4% |
|
|
[Mo2O3(C5H4NS)]+ |
353.80 |
349.96 |
3% |
Conclusion
[1] does not have any absorption in the region 2548-2602 cm-1 due to absence of S-H group in it. C=S stretching at 1261 and 1109 wave numbers are concurrent with presence of C=S group. 761 cm-1 absorption due to C-S stretching support formation of Mo-S bonds. Terminal υ(Mo=O) at 986 cm-1 is observed. S-H peak has not been observed in 1H NMR of [1]. This indicates that S-H group is absent in [1]. Fragmentation pattern in GC-MS is compatible with the proposed formula.
[2] and [3] do not have any absorption around 2670 cm-1 because there is no S-H group in them. S-H bond does not exist because of presence of υ(C=S) at 1027 cm-1 in both of them. Ions observed in mass spectrum are concurrent with the depicted formulae. S→Mo coordinate bond is likely to be present. Terminal υ(Mo=O) absorbs at 973 cm-1 in [2] and at 970 cm-1 in [3]. S-H peak is missing in 1H NMR of [2] and [3]. LC-MS supports the predicted formulae. CH3CN is present in in [2] and [3] as verified by the presence of its peak in 1H NMR.
N-H group absorbs at 3383 cm-1 in [4]. Absence of any peak around 2708 cm-1 is because of missing S-H group it. C=S stretching at 1261 cm-1, 1109 cm-1 are due to C=S group. C-S stretching at 767 cm-1, 707 cm-1 depict Mo-S bond existence. υ(Mo=O) absorption at 983 cm-1 in [4] suggests presence of terminal Mo=O group it. Fragmentation pattern in LC-MS support the proposed formula.
Acknowledgement
We thank Panjab University, Chandigarh (India) for providing testing facility.
Conflict of Interest
The authors do not have any conflict of interest.
References
- https://medlineplus.gov/druginfo/meds/a682653.html
- https://en.wikipedia.org/wiki/Mercaptopurine#:~:text=Mercaptopurine%20(6%2DMP)%2C,Crohn’s%20disease%2C%20and%20ulcerative%20colitis.
- Łakomska, I.; Pazderski, L.; Sitkowsk, J.; Kozerski, L.; Pełczynska, M.; Nasulewicz, A.; Opolski, A.; Szłyk, E., J. Mol. Struct., 2004, 707, 241-247.
CrossRef - Blank, C.; Dabrowiak, J., J. Inorg. Biochem.,1984, 21, 21-29.
CrossRef - Cuin, A.; Massabni, A. C.; Pereira, G. A.; Leite, C. Q. F.; Pavan, F. R.; Sesti-Costa, R.; Heinrich, T. A.; Costa-Neto, C. M., Biomed. Pharmacother, 2011, 65, 334-338.
CrossRef - Bariyanga, J.; Luyt, A., J. Mol. Struct.,2001, 559, 49-54.
CrossRef - Cini, R.; Cinquantini, A.; Sabat, M.; Marzilli, L. G., Inorg. Chem., 1985, 24, 3903-3908.
CrossRef - Dubler, E.; Gyr, E., Inorg. Chem.,1988, 27, 1466-1473.
CrossRef - Abeer A. Sharfalddin,; Emwas, A. H.; Jaremko, M.; Hussien, M. A.,Appl Organomet Chem.,2021, 35, 1-18, DOI: 10.1002/aoc.6041.
CrossRef - Anita M. Owczarzak, A. M.; Kubicki, M., Acta Crystallographica, Section E., 2012, E68, o1686. doi:10.1107/S1600536812020090.
CrossRef - Al-R. K. A.; Abdel-Aziz, H. A., Molecules, 2010, 15, 3775-3815.
CrossRef - Borhani, D. W.; Calderwood, D. J.; Frank, K. E. H.; Davis, M.; Josephsohn, N. S.; Skinner, B. S. WO Pat. 2008/063287, 2008.
- Fidanze, S. D.; Erickson, S. A.; Wang, G. T.; Mantei, R.; Clark, R. F.; Sorensen, B. K.; Bamaung, N. Y.; Kovar, P.; Johnson, E. F.; Swinger, K. K.; Stewart, K. D.; Zhang, Q.; Tucker, L. A.; Pappano, W. N.; Wilsbacher, J. L.; Wang, J., Sheppard, G. S.; Bell, R. L.; Davidsen, S. K.; Hubbard, R. D., Bioorg. Med. Chem. Lett., 2010, 20, 2452-2455.
CrossRef - Emmitte, K. A.; Wilson, B. J.; Baum, E. W.; Emerson, H. K.; Kuntz, K. W.; Nailor, K. E.; Salovich, J. M.; Smith, S. C.; Cheung, M.; Gerding, R. M.; Stevens, K. L.; Uehling, D. E.; Jr. Mook, R. A.; Moorthy, G. S.; Dickerson, S. H.; Hassell, A. M.; Leesnitzer, M. A.; Shewchuk, L. M.; Groy, A.; Rowand, J. L.; Anderson, K.; Atkins, C. L.; Yang, J.; Sabbatini, P.; Kumar, R. Bioorg. Med. Chem. Lett., 2009, 19, 1004-1007.
CrossRef - Andreani, A.; Burnelli, S.; Granaiola, M.; Guardigli, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Rizzoli, M.; Varoli, L.; Roda, A. Eur. J. Med. Chem., 2008, 43, 657-661.
CrossRef - Andreani, A.; Rambaldi, M.; Mascellani, G.; Rugarli, P., Eur. J. Med. Chem. 1987, 22, 19-22.
CrossRef - Gupta, G. D., Jain, K. K., Gupta, R. P., Pujari, H. K., Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1983, 22, 268.
CrossRef - Amarouch, H., Loiseau, P. R., Bacha, C., Caujolle, R., Payard, M., Loiseau, P. M., Bories, C., Gayral, P., Eur. J. Med. Chem., 1987, 22, 463-466.
CrossRef - Andreani, A.; Rambaldi, M.; Andreani, F.; Bossa, R.; Galatulas, I., Eur. J. Med. Chem. 1988, 23, 385-389.
CrossRef - Andreani, A.; Rambaldi, M.; Locatelli, A.; Bossa, R.; Fraccari, A.; Galatulas, I., Pharm. Acta Helv., 1993, 68, 21-24.
CrossRef - Andreani, A.; Bonazzi, D.; Rambaldi, M., Arch. Pharm., 1982, 315, 451-456.
CrossRef - Andreani, A.; Rambaldi, M. M.; Locatelli, A.; Bossa, R.; Fraccari, A.; Galatulas, I., J. Med. Chem., 1992, 35, 4634-4637.
CrossRef - Ding, H.; Chen, Z.; Zhang, C.; Xin, T.; Wang, Y.; Song, H.; Jiang, Y.; Chen, Y. Xu, Y.; Tan, C. Molecules, 2012, 17, 4703-4716.
CrossRef - Abdelwareth Sarhan, A.O.; Al-Dhfyan, A.; Al-Mozaini, M. A.; Adra, C. N.; Aboul-Fadl, T. Eur. J. Med. Chem., 2010, 45, 2689-2694.
CrossRef - Vu, C. B.; Bemis, J. E.; Disch, J. S.; Ng, P. Y.; Nunes, J. J.; Milne, J. C.; Carney, D. P.; Lynch, A. V.; Smith, J. J.; Lavu, S.; Lambert, P. D.; Gagne, D. J.; Jirousek, M. R.; Schenk, S.; Olefsky, J. M.; Perni, R. B., J. Med. Chem., 2009, 52, 1275-1283.
CrossRef - Poorrajab, F.; Ardestani, S. K.; Emami, S.; Behrouzi-Fardmoghaddam, M.; Shafiee, A.; Foroumadi, A. Eur. J. Med. Chem., 2009, 44, 1758-1762.
CrossRef - Khalaj, A., Nakhjiri, M., Negahbani, A. S., Samadizadeh, M., Firoozpour, L., Rajabalian, S., Samadi, N., Faramarzi, M. A., Adipour, N., Shafiee, A., Foroumadi, A., Eur. J. Med. Chem., 2011, 46, 65-70.
CrossRef - Kumaresan, K. L. Lu, S.; Wen, Y. S. ; Hwu, J. R., Organometallics, 1994, 13, 3170–3176.
CrossRef - Nagai, K.; Carter, B. J.; Xu, J.; Hecht, S. M., J. Am. Chem. Soc., 1991, 113, 5099-5100.
CrossRef - Lobana, T. S.; Bhatia, P. K., J. Sci. Ind. Res., 1989, 48, 394-401
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D., American International Journal of Research in Science, Technology, Engineering & Mathematics, 2014, 8(2), 131-136.
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D., American International Journal of Research in Science, Technology, Engineering & Mathematics, 2015,9(1), 25-33.
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D., American International Journal of Research in Science, Technology, Engineering & Mathematics, 2015,10(4), 299-308.
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D., International Congress on Chemical, Biological and Environmental Sciences,2015, 930-942, May 7-9, Kyoto (Japan).
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D.; Kumar, R., American International Journal of Research in Science, Technology, Engineering & Mathematics, 2016, 16(1), 56-64.
- Singh, G.; Kumar, R., American International Journal of Research in Science, Technology, Engineering & Mathematics, 2018, 22(1), 01-08
- Rani, D.; Singh, G.; Sharma, S., Oriental Journal of Chemistry, 2020, 36(6), 1096-1102.
CrossRef - Mangla, V.; Singh, G., American International Journal of Research in Science, Technology, Engineering & Mathematics, 2019,26(1), 145-148.
- Mangla, V.; Singh, G., Oriental Journal of Chemistry, 2019, 35(3), 1094-1102.
CrossRef - Vogel, A. I., A text book of Quantitative Inorganic Analysis; John Wiley and Sons: New York, 1963 (Standard methods).
- Jolley, J.; Cross, W. I.; Pritchard, R. G.; McAuliffe,C. A.; Nolan, K. B., Inorganica Chimica Acta, 2001, 315, 36-43.
CrossRef - Kahn, E. S.; Rheingold, A. L.; Shupack, S. I., J. Crystallographic and Spectroscopic Research, 1993, 23 (9), 697-710.
CrossRef - Trzhtsinskaya, B. V.; Abramova, N. D., J. Sulphur Chemistry, 1991, 10(4), 389-430.
CrossRef - Liang Ying-Qiu; Zhao Wen-Yun; XU Wei-Qing, Acta Chimica Sinica, 1986, 1126, 42-47.
CrossRef - Barraclough, C. G.; Kew, D. J., Australian J. Chem., 1970, 23, 2387-2396.
CrossRef - Ward, B. G.; Stafford, F. E., Inorg. Chem.,1968, 7, 2569.
CrossRef - Bodo, H. H.; Regina, Z. Chem., 1976, 16, 407.
CrossRef - Abramenko, V. L.; Sergienko, V. S., Russian J. Inorg. Chem., 2009, 54(13), 2031-2053.
CrossRef - Ueyama, N.; Nakata, M.; Araki, T.; Nakamura, A., Chemical Soc. Japan, Chemistry Lett., 1979, 421-424.
CrossRef - Kumar, G. P.; Sanganal, J. S.; Phani, A. R.; Tripathi, S. M.; Manohara, C.; Raghavendra, H. L.; Janardhana, P. B.; Amaresha, S.; Swamy, K. B.; Prasad, R. G. S. V., Phamacological Research, 2015, 100, 47-57.
CrossRef - http://www.sigmaaldrich.com/catalog/product/aldrich/m5852?lang=en®ion=IN.
- Refat, M. S.; Farias, R. F. D., J. Serb. Chem. Soc., 2006, 71(12), 1289-1300.
CrossRef - Hanif, M.; Saddiq, A.; Hasnain, S.; Ahmad, S.; Rabbani, G.; Isab, A. A., Spectroscopy, 2008, 22, 51-56.
CrossRef - Xhang, H. L.; Evans, S. D.; Henderson, J. R.; Miles, R. E.; Shen, T., J. Phys. Chem. B, 2003, 107, 6087-6095.
CrossRef - Shpakovsky, D. B.; Banti, C. N.; Houle, G. B.; Kourkoumelis, N.; Manoli, M.; Manos, M. J.; Tasiopoulos, A. J.; Hadjikakou, S. K.; Milaeva, E. R.; Charalabopoulos, K.; Bakas, T.; Butlerd, I. S.; Hadjiliadisa, N., Dalton Trans., 2012, 41, 14568-14582.
CrossRef - Abramenko, V. L.; Sergienko,V. S.; Churakov, A. V., Russian J. Coord. Chem., 2000,26(12), 866-871.
CrossRef - Sharma, M.; Koty, A.;Srivastava, M.; Srivastava, A., J. Chinese Chemical Society, 2007, 54, 1419-1432.
CrossRef - http://www.molbase.com/en/hnmr_6857-34-7-moldata-838140.html#tabs.
- http://www.sisweb.com/referenc/tools/exactmass.htm.
- https://webbook.nist.gov/cgi/cbook.cgi?ID=C7719097&Mask=28F.

This work is licensed under a Creative Commons Attribution 4.0 International License.









