Antioxidant Studies of Some Lanthanide Complexes Derived from Curcuminoid Analogues
Malini Parakkulangara Thrithody1 and Muhammed Basheer Ummathur2*
and Muhammed Basheer Ummathur2*
1Department of Chemistry, Zamorin’s Guruvayurappan College, Calicut-673014, (Kerala) India.
2Department of Chemistry, KAHM Unity Women’s College, Manjeri-676122, (Kerala) India.
Corresponding Author E-mail: mbummathur@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370110
Article Received on : 30-Nov-2020
Article Accepted on :
Article Published : 16 Feb 2021
Antioxidant activities of the Pr(III), Nd(III), Sm(III), Dy(III), Er(III) and Yb(III)complexes of eight curcuminoid analogues (HL1 to HL8), derived from acetylacetone and aromatic aldehydes (benzaldehyde, cinnamaldehyde, furfural, salicylaldehyde, β-hydoxy-α-naphthaldehyde, p-anisaldehyde, p-hydroxybenzaldehyde and vanillin), are studied by the thiocyanate method. Even though all the complexes exhibited significant antioxidant properties, their activities are found to be less than the corresponding free curcuminoid analogues.
KEYWORDS:Antioxidant Studies; Curcuminoid Analogues, Pr(III), Nd(III), Sm(III), Dy(III), Er(III) and Yb(III)Complexes; Thiocyanate Method
Download this article as:| Copy the following to cite this article: Thrithody M. P, Ummathur M. B. Antioxidant Studies of Some Lanthanide Complexes Derived from Curcuminoid Analogues. Orient J Chem 2021;37(1). |
| Copy the following to cite this URL: Thrithody M. P, Ummathur M. B. Antioxidant Studies of Some Lanthanide Complexes Derived from Curcuminoid Analogues. Orient J Chem 2021;37(1). Available from: https://bit.ly/3s13dB0 |
Introduction
Metal complexes of many plant products are receiving much importance in recent years due to their applications in various fields1-3. The metal complexes of curcumin, a naturally occurring conjugated diketone, have been studied in detail in view of its structure and medicinal applications4. Synthetic curcuminoid analogues and their metal chelates have also been investigated by various research groups due to their applications in various biological fields5-11. Majority of these works are based on the complexes with various main group and transition metal ions. The biological applications of the lanthanide chelates of synthetic curcuminoids are not studied in detail as revealed in the literature survey. In continuation of the studies on the metal complexes of curcuminoids6-11, in this paper we report the antioxidant activities of Pr(III), Nd(III), Sm(III), Dy(III), Er(III) and Yb(III)complexes of curcuminoid analogues.
Materials and Methods
All the chemicals and solvents used for the synthesis of curcuminoid analogues and their Pr(III), Nd(III), Sm(III), Dy(III), Er(III) and Yb(III)complexes were of reagent grade (Merck, Fluka and Sigma-Aldrich). For the preparation of metal complexes, Pr(III), Nd(III), Sm(III), Dy(III), Er(III) and Yb(III)nitrates were used. The antioxidant activities were studied by measuring the absorbance using a Bausch & Lomb Spectronic 1001 UV-Visible Spectrophotometer.
Synthesis of curcuminoid analogues and their complexes with Pr(III), Nd(III), Sm(III), Dy(III), Er(III) and Yb(III) ions
The curcuminoid analogues (HL1–HL8) were synthesized by condensing aromatic aldehydes with acetylacetone by the methods reported in our previously published papers6-11. The aromatic aldehydes used for the condensation were benzaldehyde, cinnamaldehyde, furfural, salicylaldehyde, β-hydroxy-α-naphthaldehyde, p-methoxybenzaldehyde, p-hydroxybenzaldehyde and vanillin.
Pr(III), Nd(III), Sm(III), Dy(III), Er(III) and Yb(III) complexes of these curcuminoid analogues were also prepared by our earlier reported methods12-13.
Determination of antioxidant activities
The thiocyanate method14,16 was employed for determining the antioxidant activity.
Results and Discussion
The structure of the lanthanide chelates of curcuminoid analogues (Fig. 1) was reported earlier12,13,15 based on various analytical and spectral techniques.
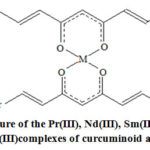 |
Figure 1: Structure of the Pr(III), Nd(III), Sm(III), Dy(III), Er(III)and Yb(III) complexes of curcuminoid analogues. |
Fig 1. Structure of the Pr(III), Nd(III), Sm(III), Dy(III), Er(III) and Yb(III)complexes of curcuminoid analogues; L stands for the deprotonated ligand; Ar = phenyl (L1);styryl (L2);2-furyl (L3);2-hydroxyphenyl (L4);2-hydroxy-1-naphthyl (L5); 4-methoxyphenyl (L6);4-hydroxyphenyl (L7);and4-hydroxy-3-methoxyphenyl (L8); M = Pr(III), Nd(III), Sm(III), Dy(III), Er(III) and Yb(III); The proposed structure also contains one bidentate nitrate ion and two coordinated water molecules with the formula [M(L)2(NO3)(H2O)2].
The antioxidant assays of lanthanide chelates of curcuminoid analogues are shown graphically (absorbance at 500 nm Vs number of days) in Fig. 2 to Fig. 9.
 |
Figure 2: Antioxidant assay of HL1 and its lanthanide chelates. |
 |
Figure 3: Antioxidant assay of HL2 and its lanthanide chelates. |
 |
Figure 4: Antioxidant assay of HL3 and its lanthanide chelates. |
 |
Figure 5: Antioxidant assay of HL4 and its Er(III) complex. |
 |
Figure 6: Antioxidant assay of HL5 and its lanthanide chelates. |
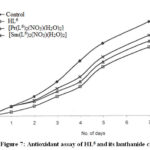 |
Figure 7: Antioxidant assay of HL6 and its lanthanide chelates. |
 |
Figure 8: Antioxidant assay of HL7 and its lanthanide chelates. |
 |
Figure 9: Antioxidant assay of HL8 and its lanthanide chelates. |
The findings showed that all lanthanide chelates possess significant antioxidant activity. A comparison with the corresponding reported curcuminoid analogues16 indicates that the antioxidant activities of these lanthanide chelates are less than the corresponding free curcuminoids.
It was reported that curcuminoids having a hydroxyl group in the aryl ring show maximum antioxidant activity6-8,10,16,17. But the results revealed that Pr(III), Nd(III), Sm(III), Dy(III), Er(III) and Yb(III)complexes of curcuminoids which contain a hydroxyl group in the aryl ring also showed lower activity than the corresponding curcuminoid analogues. Thus it can be inferred that the –OH group alone is not a sufficient condition to promote antioxidant activity. This indicates the importance of free and enolised dicarbonyl moiety in imparting the antioxidant property of curcuminoids.
During complexation, the enolic proton is removed with the formation of a six membered pseudo-aromatic C3O2M chelate ring with the metal ion, thereby losing the enolic nature of curcuminoids. Earlier reports of certain transition metal complexes of curcuminoids also showed lesser activity than the corresponding curcuminoids18. It has been reported that the antitumour activity of curcuminoids increases on complexation with Cu(II) and Al(III) ions6-10. The results of the present investigation clearly indicate that the antioxidant activity of curcuminoids decreases on complexation with Pr(III), Nd(III), Sm(III), Dy(III), Er(III) and Yb(III)ions.
Conclusion
The effects of complexation of Pr(III), Nd(III), Sm(III), Dy(III), Er(III) and Yb(III)ions on the antioxidant properties of curcuminoid analogues were studied. Even though all the lanthanide chelates exhibited significant antioxidant activity, the metal complexation lowered the activities of all the curcuminoids. The results revealed that the enolised and intramolecularly hydrogen bonded dicarbonyl moiety is mainly responsible for the antioxidant property of all curcuminoids. The decrease in the antioxidant property during complex formation arises due to the replacement of this intramolecularly hydrogen bonded enolic proton by metal cation.
Acknowledgement
The authors express sincere thanks to University Grants Commission, New Delhi, India for providing financial assistance; and Department of Life Sciences, University of Calicut, India for providing facilities for the antioxidant studies.
Conflicts of Interest
No conflict of interest regarding this article.
References
- Castillo-Blum, S. E.; Barba-Bahrens, N. Coord. Chem. Rev. 2000,196(1), 3-30.
CrossRef - Singh, R. V.; Srivastava, S. J. Ind. Chem. Soc. 2013, 90(6), 711-737.
- Cotruvo, J. A. ACS Cent. Sci. 2019,5(9), 1496-1506.
CrossRef - Shakeri, A.; Panahi, Y.; Johnston, T. P.; Sahebkar, A. BioFactors. 2019,45(3), 304-317.
CrossRef - Călinescu, M.; Fiastru, M.; Bala, D.; Mihailciuc, C.; Negreanu-Pîrjol, T.; Jurcă, B. J. Saudi Chem. Soc. 2019,23(7), 817-827.
CrossRef - John, V. D.; Ummathur, M. B.;Krishnankutty, K. J. Coord. Chem. 2013, 66(9), 1508-1518.
CrossRef - John, V. D.; Krishnankutty, K. Appl. Organometal. Chem.2006,20(8), 477-482.
CrossRef - John, V. D.; Krishnankutty, K. Trans. Met. Chem.2005,30, 229-233.
CrossRef - Krishnankutty, K.; John, V. D.Synth. React. Inorg. Met.-Org. Chem.2003,33, 343-358.
CrossRef - John, V. D.; Kuttan, G.; Krishnankutty, K. J. Exp. Clin. Cancer Res.2002, 21(2), 219-224.
- Krishnankutty, K.; Venugopalan, P. Synth. React. Inorg. Met-Org. Chem. 1998,28,1313-1325.
CrossRef - Malini, P. T.; Ummathur, M. B. J. Appl. Sci. Comput. 2020, 7(9), 72-75.
- Malini, P. T.; Ummathur, M. B.; Krishnankutty, K. J. Appl. Sci. Comput. 2020, 7(10), 163-166.
- Ak, T.; Gülçin, I. Chem. Biol. Interact.2008,174(1), 27-37.
CrossRef - Malini, P. T.; Ummathur, M. B.; Krishnankutty, K. J. Appl. Sci. Comput. 2020, 7(11), 10-13.
- Malini, P. T.; Ummathur, M. B.; Krishnankutty, K. Int. J. Recent Sci.2020,11, 40089-40091.
- Weber, W. M.; Hunsaker, L. A.; Abcouwer, S. F.; Deck, L. M.; Vander Jagt, D. L. Bioorg. Med. Chem. 2005, 13(11), 3811–3820.
CrossRef - Priya, R. S.; Balachandran, S.; Joseph, D.; Mohanan, P. V. Univ. J. Phys. Appl.2015,3(1), 6-16.

This work is licensed under a Creative Commons Attribution 4.0 International License.









