Synthesis, Characterization of Copper Complexes of 9H-Carbazole-3-carbaldehyde-4-Phenylthiosemicarbazone, 10-Hexyl-10-H-phenothiazine-3-carbaldehyde-4-Phenylthio semicarbazone and 2-Thiophenecarboxaldehyde-4-methylthiosemicarbazone and Anti-bacterial Activity Studies of Ligands and Complexes
N. Rama Jyothi1,2, N.A. Mohamed Farook1, Jaya Madhuri3 and K. Gowthami 3
1Department of Chemistry, Khadir Mohideen College, Adhirampattinam, Tamil Nadu- 614 701, India.
2Department of Chemistry, College of Engineering, Sri Padmavathi Mahila Visvavidyalayam,Tirupati, Andhra Pradesh - 517 501, India.
3Department of Microbiology, Sri Padmavathi Mahila Visvavidyalayam, Tirupati, Andhra Pradesh - 517 501. India.
Corresponding Author E-mail: ramadasaradhi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360615
Article Received on : 09-10-2020
Article Accepted on :
Article Published : 30 Dec 2020
In Coordination chemistry metal chelating agents has a vital role, among them thiosemicarbazones occupies an important place due to their a range of applications in different fields, such as analytical and biological. In the literature we can find many thiosemicarbazones which has a wide range of applications both in pharmacy and chemical fields. But still there is a scope is there to synthesize new thiosemicarbazone ligands and their metal complexes due to their utility is still there in modern chemistry also. In this present study we synthesized the copper(II) complexes of 9H-Carbazole-3-carbaldehyde-4-phenylthiosemicarbazone, 10-hexyl-10-H-phenothiazine-3-carbaldehyde-4-phenylthiosemicarbazone, and 2-thiophenecarboxalde -hyde-4-methylthiosemicarbazone and these complexes are characterized with FT-IR, XRD analysis and thermal stabilities of the both ligands and complexes are compared with thermogravimetric analysis studies. Finally, the antibacterial activities of both chelating agents and their metal complexes are tested with two gram positive bacterial stains, such as Bacillus subtilis, Staphylococcus aureusand two gram negative bacterial stains, Pseudomonas aeruginosa, Escherichia coli.
KEYWORDS:Antibacterial Studies; Copper(II) Complexes; 9H-Carbazole-3-Carbaldehyde-4-Phenylthiosemi Carbazone; 10-Hexyl-10-H-Phenothiazine-3-Carbaldehyde-4-Phenyl Thiosemicarbazone; 2-Thiophenecarboxaldehyde-4-Methylthiosemicarbazone
Download this article as:| Copy the following to cite this article: Jyothi N. R, Farook N. A. M, Madhuri R. J, Gowthami K. Synthesis, Characterization of Copper Complexes of 9H-Carbazole-3-carbaldehyde-4-Phenylthiosemicarbazone, 10-Hexyl-10-H-phenothiazine-3-carbaldehyde-4-Phenylthio semicarbazone and 2-Thiophenecarboxaldehyde-4-methylthiosemicarbazone and Anti-bacterial Activity Studies of Ligands and Complexes. Orient J Chem 2020;36(6). |
| Copy the following to cite this URL: Jyothi N. R, Farook N. A. M, Madhuri R. J, Gowthami K. Synthesis, Characterization of Copper Complexes of 9H-Carbazole-3-carbaldehyde-4-Phenylthiosemicarbazone, 10-Hexyl-10-H-phenothiazine-3-carbaldehyde-4-Phenylthio semicarbazone and 2-Thiophenecarboxaldehyde-4-methylthiosemicarbazone and Anti-bacterial Activity Studies of Ligands and Complexes. Orient J Chem 2020;36(6). Available from: https://bit.ly/2Vg3ZvB |
Introduction
Organic chelating agents plays an important role in both analytical and biological fields. Among organic reagents, thiosemicarbazones have a vital role due to the ability of formation of bonds with enzymes in biological systems. The donor atoms of nitrogen and sulphur of thiosemicarbazones forms a chelating bonds with the wide range of metal ions [1-4]. In recent years, so many authors were reported regarding the synthesis and characterization studies of thiosemicarbazones and their metal complexes. But still its potentiality has not been exposed yet. Its owes us to synthesize new thiosemicarbazone metal complexes and to study their biological activities. Metal complexes of thiosemicarbazones were proven as anti-bacterial [5,6,7], anti-fungal [8,9], anti-cancer [10,11] agents. Chandra and Vandana [12] had reported the anti-bacterial and anti-cancer activities of copper and nickel complexes of 2-carboxybenzaldehyde thiosemicarbazone. Khan et al. [13] reported about the synthesis, characterization and antibacterial properties of thiosemicarbazone ligand and their copper, nickel, and cobalt complexes. Piri et al. [14] reported the anti-oxidant and anti-bacterial activities of Cu(II) complexes of thiosemicarbazone ligands.
A recent study [15] reported the antibacterial activity of cinnamaldehydethiosemicarbazones against the bacterial stains, such as E. coli and K.pneumoniae. In this study they tested the antibacterial activity of thiosemicarbazone ligands and their Cu(II) and Zn(II) complexes. This study found that the compound which has lowest partition co-efficient has more antibacterial activities on the tested bacteria stains. Based on the results they concluded that the metalloantibiotics are a reliable compounds in fighting against the bacterial stains. Copper, cobalt and nickel metal complexes of coumarine-3- ylthiosemicarbazone compounds were tested for antibacterial properties towards few gram +ve and gram -ve bacterial stains by Refatetal. [16]. Naveen et al. [17] reported about the study related to substituted triazolethiosemicarbazone derivatives preparation and their biological activities. In this study they found that few of their tested compounds were had more potential activity against B. subtilis and P. aeruginosa than a standard reference drug, Ciprofloxacin. The antibacterial studies performed in this study was supported by molecular docking studies also.
Kumar et al. [18] reported the synthesis and antibacterial activities of Co(II), Cu(II), Ni(II), and Zn(II) complexes of acenaphthoquinone 3-(4-benzylpiperidyl) thiosemicarbazones against Escherichia coli, Pseudomonas aeruginosa and S.aureus and B.subtilis. In this study they reported Ni(II) complexes had greater activity than other three metal complexes against the tested bacterial stains. Antibacterial and anti-tumor activities of diorganotin complexes with 3-methoxy salicylaldehydethiosemicarbazone was reported by Khandani et al. [19]. They tested the chelating agents and their metal complexes towards the bacteria stains, reported that one of the metal complex shows comparative activity along with the standard drugs.
Akbari et al [20] studied the antibacterial activities of Ni(II) complexes of isothiosemicarbazone and thiosemicarbazone ligands. This study reports one of the Ni(II) complexes had higher antibacterial property against the Staphylococcus aureus than other complexes. The inhibition zone and minimal inhibition concentration of Ni(II) complex with higher activity was found that 30mm, and 1.56 mg/mL, respectively.
The present study reports the synthesis and characterization of copper(II) complexes of 9H-Carbazole-3-carbaldehyde-4-phenylthiosemicarbazone (Cu-CCPTSC), 10-Hexyl-10-H-phenothiazine-3-carbaldehyde-4-phenylthiosemicarbazone (Cu-HPCPTSC), 2-Thiophenecarboxalde -hyde-4-methylthiosemicarbazone (Cu-TCMTSC) with FT-IR, XRD and thermogravimetric analysis. Antibacterial activities of both chelating agents and their coppercomplexes against two gram positive bacterial stains, Bacillus subtilis, Staphylococcus aureusand two gram negative bacterial stains, Pseudomonas aeruginosa, Escherichia coli.
Experimental
Synthesis of ligands
The ligands which are used for the chelation with copper metal ions, such as 9H-carbazole-3-carbaldehyde-4-phenyl-3-thiosemicarbazone (CCPTSC), 10-Hexyl-10-H-phenothiazine-3-carb -aldehyde-4-phenylthiosemicarbazone (HPCPTSC) and 2-Thiophenecarboxaldehyde-4-methylthiosemicarbazone (TCMTSC) were synthesized as per literature [21,22].
Instrumentation
Infrared Spectrum
The FT-IR spectra were recorded on a Nicolet FT-IR 560 Magna spectrometer.
XRD Spectra
Powder XRD (PAN analytical X’Pert PRO, USA) was carried out using CuKα (0.154056 nm) radiation at 40 kV and 30 mA. The data was collected between 10 and 70°2θ with a step size of 0.02°.
Thermogravimetric Analysis
Thermogravimetric analysis was carried out in the temperature range of 25-800° C in nitrogen atmosphere and a heating range of 10° C/min using SDT Q600 V20.9 Build 20 (TA instruments, Waters, USA).
Preparation of copper(II) complex of 9H-carbazole-3-carbaldehyde-4-phenyl-3-thiosemicarbazone (Cu-CCPTSC)
Methanolic solutions of 0.34 g of 9H-carbazole-3-carbaldehyde-4-phenyl-3-thiosemicarba -zone in 100 mLand 0.09g of copper(II) chloride dihydrate in 100 mL were refluxed about 4 h. in a flask. Obtained grass green product (with yield, 86%) which is then separated through the filtration. This filtered product was then dried. To obtain the pure product it was then recrystallized with methanol. A possible structure of copper(II) complex of 9H-carbazole-3-carbaldehyde-4-phenyl-3-thiosemicarbazone is presented in scheme 1.
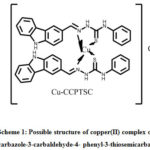 |
Scheme 1: Possible structure of copper(II) complex of 9H-carbazole-3-carbaldehyde-4- phenyl-3-thiosemicarbazone |
Preparation of copper(II) complex of HPCPTSC
Methanolic solutions of 0.23 g of HPCPTSC in 100 mLand 0.045 g of copper(II) chloride dihydrate in 100 mL were refluxed for nearly 4 h. in a RB flask. Obtained grey product(with an yield, 89%) was separated through the filtration and dried over. Final product then recrystallizes from methanol. A possible structure of copper(II) complex of HPCPTSC is presented in scheme 2.
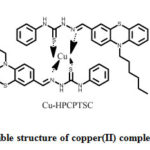 |
Scheme 2: Possible structure of copper(II) complex of HPCPTSC |
Preparation of copper(II) complex of 2-thiophenecarboxaldehyde-4-methyl-3-thiosemicarbazone(Cu-TCMTSC)
Copper(II) complex of 2-thiophenecarboxaldehyde-4-methyl-3-thiosemicarbazone has synthesizes by heating an 50mL methanolic2-thiophene carboxaldehyde-4-methyl-3-thiosemicarbazone(0.40 g) and 0.18 g of copper(II) chloride dihydrate in 50 mL of methanolfor nearly 4 and half an hour in a RB flask. A persistent blue product was formed(with an yield, 81%) has took off through filtration, which was then dried. Thenfinal product recrystallizes with methanol. A possible structure of copper(II) complex of 2-thiophene carboxaldehyde-4-methyl-3-thiosemicarbazone is presented in scheme 3.
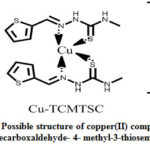 |
Scheme 3: Possible structure of copper(II) complex of 2-thiophenecarboxaldehyde- 4- methyl-3-thiosemicarbazone |
Experimental procedure for anti-bacterial activity
Antibacterial properties of the ligands (1-9) and their complexes (1A-9A) screened towards two gram +ve bacterial stains, B.subtilis, S.aureusand two gram -ve bacterial stainsP.aeruginosa, E. coli using agar well diffusion method26. Stock solution was prepared by dissolving 1 mg of the test compounds in 10 mL of dimethyl sulphoxide and further diluted to prepare 100 μg/mL concentrations. The antibiotic Tetracycline was used as standard for comparing activity of the tested compounds. Freshly prepared Muller Hinton Agar medium (20 mL) was poured in each Petri plate.After solidification of the medium, 24 h cultures were prepared for micro-organisms B.subtilis, S.aureus, P. aeruginosa and E. coli containing approximately 105 – 106 colony forming units (CFU) per mL was spread on the surface of the medium.Sterile swabs are used to swabin the culture on nutrient agar plate for making microbial lawn on sterile nutrient agar plate. Sterile filter papers were placed on laun. The different concentration of compounds are 25, 50, 75 and 100 μLand positive and negative control were placed on the disc. After 24h inhibition of the test pathogens by the synthesized compounds around the wells was measured to determine the antibacterial (zone of inhibition) activity of the samples were studied to know the potency. The experiments were conducted in triplicate and average tabulated as final result.
Result and Discussion
Characterization of Cu-CCPTSC, Cu-HPCPTSC and Cu-TCMTSC
Copper(II) complexes of CCPTSC, HPCPTSC and TCMTSC are characterized with FT-IR, XRD. Thermogravimetric studies were performed to compare the thermal stabilities of ligands and their metal complexes.
FT-IR analysis of Cu-CCPTSC, Cu-HPCPTSC and Cu-TCMTSC
FT-IR spectra of free ligands CCPTSC, HPCPTSC and TCMTSC are presented in our previous studies [21,22]. By comparing the spectra of complexes with free ligands it is confirmed the chelation of metal ion with free ligands.
FT-IR spectrum of complex Cu-CCPTSCis presented in Figure 1. The IR spectra of complex 4A is represented as: Carbon-Nitrogen peak appears at 1581 cm-1 and Carbon-Sulphur peak at 1151 cm-1. When this data compared with its parent ligand, Carbon-Nitrogen peak is changed from 1635 to 1581 cm-1.Carbon-Sulphur peak is changed from 1222 to 1151 cm-1, this change is due to complexation of metal with chelating agent. This shift confirms the formation of the complex Cu-CCPTSC.
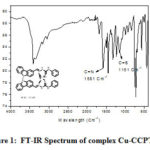 |
Figure 1: FT-IR Spectrum of complex Cu-CCPTSC Click here to View figure |
FT-IR spectrum of complex HPCPTSC is presented in Figure 2. The spectral data of complex 5Ais represented as Carbon-Nitrogen peak appears at 1558 cm-1 and Carbon-Sulphur peak appears at 1148 cm-1. When this data compared with its parent ligandCarbon-Nitrogen peak is changed from 1625 to 1558 cm-1 and Carbon-Sulphur peak is changed from 1236 to 1148 cm-1, this change is due to complexation of metal with chelating agent. This dataindicates the formation of complex Cu- HPCPTSC.
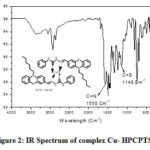 |
Figure 2: IR Spectrum of complex Cu- HPCPTSC |
In Figure 3 IR spectrum of complex Cu-TCMTSCis presented. IR spectra of complex Cu-TCMTSC is presented as, Carbon-Nitrogen peak appears at 1581 cm-1 and Carbon-Sulphur peak appears at 1173 cm-1. When this data compared with its parent ligand, C=N peak is shifted from 1624 to 1581 cm-1 and C=S peak is shifted from 1232 to 1173 cm-1, this shift is due to the chelation of metal ion to the chelating agent. This shift confirms the formation of the complex Cu-TCMTSC.
 |
Figure 3: FT-IR Spectrum of complex Cu-TCMTSC |
XRD analysis of Cu-CCPTSC, Cu-HPCPTSC and Cu-TCMTSC
XRD studies were performed to identify crystalline nature of copper(II) complexes of thiosemicarbazone ligands. Figures 4 to 6 represents the XRD spectra of the complexes Cu-CCPTSC, Cu-HPCPTSC and Cu-TCMTSC, respectively. From the diffractograms it is observed that the dominant 2θ XRD peaks for the complexes Cu-CCPTSC, Cu-HPCPTSC and Cu-TCMTSC are appears at 22.30°, 16.19° and 6.33, respectively. Except Cu-CCPTSC, in all the remaining complexes the dominant 2θ XRD peaks are shifted to lower side than their ligands. The morphology of ligands has completely changed due to the chelation with copper(II) ion.
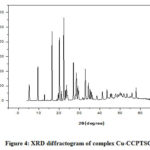 |
Figure 4: XRD diffractogram of complex Cu-CCPTSC Click here to View figure |
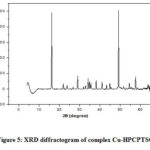 |
Figure 5: XRD diffractogram of complex Cu-HPCPTSC. |
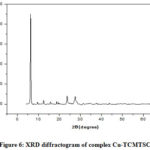 |
Figure 6: XRD diffractogram of complex Cu-TCMTSC. |
Thermogravimetric analysis of Cu-CCPTSC, Cu-HPCPTSC and Cu-TCMTSC
The thermo gravimetric curve of complex Cu-CCPTSC is presented in Figure 7. The thermo gravimetric curve of complex Cu-CCPTSC shows that the complexstarts melting at 100°C, the decomposition observed at 250°C and 525°C with a weight loss of 36% and56%, respectively and almost 63.5% weight loss observed at 800°C by remaining 36.5% of the complex.
 |
Figure 7: TGA curve of complex Cu-CCPTSC. |
The thermo gravimetric curve of complexCu-HPCPTSC is presented in Figure 8. From the thermo gravimetric curve of complexCu-HPCPTSC, it is observed that the complex starts melting at 260°C and the decomposition observed at 490°C with a weight loss of 60% and more than 65% weight loss observed at 800°C. The thermo gravimetric curve of complex Cu-TCMTSC indicates that the compound starts melting at 210°C and the decomposition observed at 325°C with a weight loss 58% and more than 77% weight loss observed at 800°C.
 |
Figure 8: TGA curve of complex Cu-HPCPTSC Click here to View figure |
Thermo gravimetric curve of complex Cu-TCMTSCis presented Figure 9.The thermal stability of the complexes is follows the order Cu-CCPTSC > Cu-HPCPTSC > Cu-TCMTSC. When compare the thermal stabilities of thiosemicarbazone ligands (1-9) and their copper(II) complexes (1A-9A) the following points are predicted.
 |
Figure 9: TGA curve of complex Cu-TCMTSC |
The complex Cu-CCPTSC remained at 800°C is 31% more than its parent ligand at the same temperature. The thermal stability of the ligand is increases with the chelation of metal ion. This is may be attributed to the formation of complex which has the increase in molecular weight and also the formation of metal to ligand bond also takes part in the increase in the thermal stability of the complex. The complex Cu-HPCPTSC remained at 800°C is 33% more than its parent ligand at the same temperature.The complex Cu-TCMTSC remained at 800°C is 21% more than its parent ligand at the same temperature.
From the above, it is concluded that all the complexes are having greater thermal stabilities than their parent ligands. Among the complexes, which are formed from phenylthiosemicarba zone ligands are having greater stability than the complex formed from methylthiosemicarbazone ligand. Both the complexes formed from phenylthiosemicarbazone ligands are remained nearly 40% at 800°C. But the complex formed from methylthiosemicarbazone ligand is left only 20% after 800°C. Based on this it is assumed that phenyl group presence also takes part in the increase in the thermal stability of the compounds. The presence of the metal ion also one of the reasons for the increase in the thermal stability of the ligand. The stronger the metal to ligand bond may also enhances the thermal stability of the ligand. Copper metal ions shows greater affinity for thiosemicarbazone ligands due to the presence of sulphur atom.
Antibacterial studies of chelating agents and their copper complexes
To evaluate antibacterial properties of newly synthesized nine chelating agents and their complexes with copper towards two gram +ve bacteria, B.subtilis, S.aureus and two gram -ve bacteria, P.aeruginosa, E.coli. For this purpose four different concentrations ( 25, 50, 75 and 100 µL) of chelating agents and their copper complexes were used. The data obtained in this study is presented in Table 1. Based on the data obtained in this study, the order of reactivity of nine chelating agents and their copper complexes is presented in Table 2.
Table 1: Minimal inhibition zones (in mm) of ligands (and their copper(II) complexes against four bacterial stains at four different concentrations
|
Concentration ( µL) |
B.subtilis |
S.aureus |
P.aeruginosa |
E. coli |
||||||||||||
|
25 |
50 |
75 |
100 |
25 |
50 |
75 |
100 |
25 |
50 |
75 |
100 |
25 |
50 |
75 |
100 |
|
|
CCPTSC |
8.0 |
18.0 |
21.0 |
25.0 |
6.0 |
12.0 |
15.0 |
19.0 |
8.0 |
15.0 |
17.0 |
21.0 |
2.0 |
5.0 |
7.0 |
9.0 |
|
Cu-CCPTSC |
14.0 |
19.0 |
20.0 |
28.0 |
10.0 |
22.0 |
26.0 |
30.0 |
14.0 |
22.0 |
25.0 |
31.0 |
8.0 |
13.0 |
19.0 |
21.0 |
|
HPCPTSC |
7.0 |
13.0 |
18.0 |
25.0 |
8.0 |
14.0 |
18.0 |
22.0 |
3.0 |
10.0 |
12.0 |
24.0 |
4.5 |
9.0 |
13.0 |
16.0 |
|
Cu-HPCPTSC |
11.0 |
14.0 |
20.0 |
26.0 |
18.0 |
20.0 |
25.0 |
31.0 |
5.0 |
12.0 |
14.0 |
25.0 |
10.0 |
19.0 |
22.0 |
24.0 |
|
TCMPTSC |
4.0 |
9.0 |
11.0 |
17.0 |
6.0 |
10.0 |
14.0 |
17.0 |
5.0 |
10.0 |
14.0 |
21.0 |
5.0 |
9.0 |
10.0 |
12.0 |
|
Cu-TCMTSC |
10.0 |
12.0 |
14.0 |
21.0 |
18.0 |
20.0 |
22.0 |
26.0 |
8.0 |
12.0 |
19.0 |
25.0 |
13.0 |
17.0 |
20.0 |
25.0 |
Table 2: Order of reactivity of ligands and their copper(II) complexes against four bacterial stains at four different concentrations
|
S.No. |
Conc. (µL) |
Compounds |
Order of reactivity |
|
1 |
25 |
CCPTSC |
B. subtilis=P. aeruginosa>S. aureus>E.coli |
|
Cu-CCPTSC |
B. subtilis=P. aeruginosa>S. aureus>E.coli |
||
|
50 |
CCPTSC |
B. subtilis>P. aeruginosa>S. aureus>E.coli |
|
|
Cu-CCPTSC |
S. aureus=P. aeruginosa>B. subtilis>E.coli |
||
|
75 |
CCPTSC |
B. subtilis>P. aeruginosa>S. aureus>E.coli |
|
|
Cu-CCPTSC |
S. aureus>P. aeruginosa>B. subtilis>E.coli |
||
|
100 |
CCPTSC |
B. subtilis>P. aeruginosa>S. aureus>E.coli |
|
|
Cu-CCPTSC |
P. aeruginosa>S. aureus>B. subtilis>E.coli |
||
|
2 |
25 |
HPCPTSC |
S. aureus>B. subtilis>E.coli>P. aeruginosa |
|
Cu-HPCPTSC |
S. aureus>B. subtilis>E.coli>P. aeruginosa |
||
|
50 |
HPCPTSC |
S. aureus>B. subtilis>P. aeruginosa>E.coli |
|
|
Cu-HPCPTSC |
S. aureus>E.coli>B. subtilis>P. aeruginosa |
||
|
75 |
HPCPTSC |
B. subtilis=S. aureus>E.coli>P. aeruginosa |
|
|
Cu-HPCPTSC |
S. aureus>E.coli>B. subtilis>P. aeruginosa |
||
|
100 |
HPCPTSC |
B. subtilis>P. aeruginosa>S. aureus>E.coli |
|
|
Cu-HPCPTSC |
S. aureus>P. aeruginosa>B. subtilis=E.coli |
||
|
3 |
25 |
TCMPTSC |
S. aureus>P. aeruginosa=E.coli>B. subtilis |
|
Cu-TCMTSC |
S. aureus>E.coli>B. subtilis>P. aeruginosa |
||
|
50 |
TCMPTSC |
S. aureus=P. aeruginosa>B. subtilis=E.coli |
|
|
Cu-TCMTSC |
S. aureus>E.coli. aeruginosa=B. subtilis |
||
|
75 |
TCMPTSC |
S. aureus=P. aeruginosa>B. subtilis>E.coli |
|
|
Cu-TCMTSC |
S. aureus>E.coli>P. aeruginosa>B. subtilis |
||
|
100 |
TCMPTSC |
P. aeruginosa>B. subtilis=S. aureus>E.coli |
|
|
Cu-TCMTSC |
S. aureus>P. aeruginosa=E.coli>B. subtilis |
The antibacterial activity of parent ligand is increases with all tested four bacterial stains. In case of B. subtilis there is a very slight increase in activity is observed in ligand 4 by chelation with copper(II). In case of E.coli, there is a huge change in activity is observed in ligand 4 by chelation with metal ion (Table 2). In this case almost the activity is doubled by chelation with metal ion. In case of S. aureus and P. aeruginosa there is a uniformity of increase in activity of ligand 4 and 4A at all concentrations. The complex 5A has higher activity than its parent ligand 5 among all tested bacterial stains. The increase in activity is very marginal in case of B. subtilis and P. aeruginosa at all concentrations. But significant increase in activity is observed in S. aureus. High increase in activity is observed in case of E.coli due to chelation with copper(II). In case of B. subtilis and P. aeruginosa there is a slight increase in complex 6A compare to its parent ligand 6 is observed at all concentrations. In case of S. aureus, at low concentration (25 µL) 6A exhibits nearly 3 times activity than its parent ligand but high concentration (100µL) only 50% increase in activity is observed. In case of E.coli, at all concentrations nearly 2 times higher activity is observed for 6A than its ligand 6. Among the ligands and complexes, ligands exhibit some inhibitory biological activitywhere as complexes at higher concentration shows best activity.Minimal inhibition zones (in mm) values gives an interesting information about bacterial action towards the compound. From these studies, it is proven that metal thiosemicarbazones are having potential antibacterial activity. The reason for the activity of the metal thiosemicarbazones can be established based on structure-activity relationship studies.
Antibacterial activity of 9H-carbazole-3-carbaldehyde-4-phenyl-3-thiosemicarbazone(CCPTSC) and its copper(II) complex (Cu-CCPTSC)
Antibacterial activity of 9H-carbazole-3-carbaldehyde-4-phenyl-3-thiosemicarbazone(CCPTSC) and its copper(II) complex (Cu-CCPTSC) is presented in Figure 10.
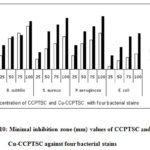 |
Figure 10: Minimal inhibition zone (mm) values of CCPTSC and Cu-CCPTSC against four bacterial stains |
The above figure indicates that chelation with copper(II) the antibacterial activity of parent ligand is increases with all tested bacterial stains. In case of B. subtilis there is a very slight increase in activity is observed in ligand CCPTSC by chelation with copper (II). In case of E.coli, there is a huge change in activity is observed in ligand CCPTSC by chelation with metal ion. In this case almost the activity is doubled by chelation with metal ion. In case of S. aureus and P. aeruginosa there is a uniformity ofincrease in activity of ligand CCPTSC and Cu-CCPTSC at all concentrations.
Antibacterial activity of HPCPTSC and its copper(II) complex (Cu-HPCPTSC)
Antibacterial activity of HPCPTSC and its copper(II) complex (Cu-HPCPTSC) is presented in Figure 11.
 |
Figure 11: Minimal inhibition zone values (mm) of HPCPTSC and Cu-HPCPTSC against four bacterial stains |
The above figure indicates that the complex Cu-HPCPTSC has higher activity than its parent ligand HPCPTSC among all tested bacterial stains.The increase in activity is very marginal in case of B. subtilis and P. aeruginosa at all concentrations. But significant increase in activity is observed in S. aureus. High increase in activity is observed in case of E.coli.
Antibacterial studies of 2-thiophenecarboxaldehyde-4-methyl-3-thiosemicarbazone (TCMTSC) and its copper(II) complex (Cu-TCMTSC)
Antibacterial study of 2-thiophenecarboxaldehyde-4-methyl-3-thiosemicarbazone(TCMTSC)and its copper(II) complex (Cu-TCMTSC) is presented in Figure 12.
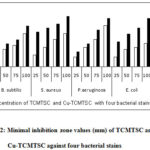 |
Figure 12: Minimal inhibition zone values (mm) of TCMTSC and Cu-TCMTSC against four bacterial stains |
The above figure indicates that chelation with copper(II) the antibacterial activity of parent ligand is increases with all tested bacterial stains. In case of B. subtilis and P. aeruginosa there is a slight increase in complex Cu-TCMTSC compare to its parent ligand TCMTSC is observed at all concentrations. In case of S. aureus, at low concentration (25 µL) 6A exhibits nearly 3 times activity than its parent ligand but high concentration (100µL) only 50% increase in activity is observed. In case of E.coli, at all concentrations nearly 2 times higher activity is observed for Cu-TCMTSC than its ligand TCMTSC.
Conclusion
From all the above, it is confirmed the synthesis of copper(II) complexes of CCPTSC, HPCPTSC and TCMTSC. All the characterization studies including FT-IR, XRD and thermogravimetric studies confirms the chelation of copper(II) with the thiosemicarbazone ligands. Based on thermogravimetric analysis studies, it is found that complexes of copper are more stable than their chelating agents. XRD studies found change in the morphology of ligands due to the chelation with copper metal ions. Antibacterial studies concludes that the complexes are having higher activity than their parent ligands against all tested bacterial stains. The activity of ligands are enhanced with the chelation of metal ion. The activity of CCPTSC and TCMTSC against E.coli almost doubled due to chelation with copper(II) ions. Cu-CCPTSC complexes shows higher activity against P. aeruginosa while other complexes are having with S. aureus. To determine the reason for increasing the activity of the free ligands by the chelation with copper(II) ions we are planning to establish the structure activity relationship studies.
Acknowledgements
One of the authors, N. Rama Jyothi is thankful to Sri Bharathidasan University, Tiruchirapalli for awarding doctor of philosophy for submitting the research work including the present study and also thankful to KhadirMohideen College, Adhirampattinam for supporting during the research work.
Conflict of Interest
Contributing authors have no conflict of interest regarding the publication of this research work
Funding Resourse
there is no funding resourses.
References
- Oliveira, C.G.; CanelonI.R.; Silva, M.M.; Coverdale, J.P.C.; Maia, P.I.S.; Batista, A.A.; Castelli, S.; Desideri, A.; Sadler, P.J.; Deflon, V.M. Dalton Trans.,2019,48 16509.
CrossRef - Adak, P.; Ghosh, B.; Bauza, A.; Frontera, A.; Herron, S.R.; Chattopadhyay, S.K. RSC Adv.,2020,10, 12735.
CrossRef - Kallus, S.; Uhlik, L.; Schoonhoven, S.V.; Pelivan, K.; Berger, W.; Enyedy, E.A.; Hofmann, T.; Heffeter, P.; Kowol, C.R.; Keppler, B.K.; J. Inorg. Biochem.,2019,190, 85.
CrossRef - Yousuf, T.A.; Abu El-Reash, G.M. J. Mol. Struct.,2020,1201, 127180.
CrossRef - Salam, M.A., Alam, M.; Sarker, S.; Rahman, M. M. J. Coord. Chem.,2018,71, 1593.
CrossRef - Khan, S.A.; Asiri, A.M. Steroids,2017,124, 23.
CrossRef - Srinivasulu, K.; Dhanalakshmi, D.; Anuja, K.; Reddy, K.H. Eur. J. Biomed. Pharm. Sci.,2019,6, 317.
- Rama Jyothi, N., Farook., N.A.M.; Vijala Lakshmi, D.; Yadav, P.S. Pharmacophore2019,10, 1.
- Chan, Y.C.; Ali, A.S. M.; Salleh, B.; Rosli, H.; Quah, C.K. Arabian J. Chem.,2017,10 53493.
CrossRef - Ouyang, R.; Yang, Y.; Tong, X.; Yang, Y.; Tao, H.; Zong, T.; Feng, K.; Jia, P.; Cao, P.; Guo, N.; Chang, H.; Zhou, S.; Mial, Y. Inorg. Chem. Commu., 2016,73, 138.
CrossRef - Parsa, F.G.; Mah, F.; Safaralizadeh, R.; Hosseini-Yazdi, S.A.; Mahdavi, M. J. Bio. Inorg.Chem.,2020. https://doi.org/10.1007/s00775-020-01769-0.
CrossRef - Chandra, S.; Vandana, Spectrochim. Acta Part A2014,129, 333.
CrossRef - Khan, S.A.; Asiri, A.M.; Amry, K.A.; MalikM.A. The Scientific World Journal2014,2014 Article ID: 592375.
CrossRef - Piri, Z.; Shoeli, Z. M.; Assoud, A. Inorg. Chem. Commun.,2017,84, 122.
CrossRef - Bisceglie, F.; Bacci, C.; Vismarra, A.; Barilli, E.; Pioli, M.; Orsoni,N.;Pelosi,G. J. Inorg. Bio Chem.,2020,203, 110888.
CrossRef - Refat, M.S.; El-Deen, I.M.; Anwer, Z.M.; El-Ghol, S. J. Mol. Struct.,2009,920, 149-162.
CrossRef - Naveen, R.K.; Tittal, V.D.; Ghule, N.; Kumar, L.; Kumar, K.; Kumar, A. J. Mol. Struct.,2020,1209, 127951.
CrossRef - Kumar, S.; Hansda, A.; Chandra,A.;Kumar, A.;Kumar, M.;Sithambaresan, M.; Faizi, Md. S. H.; Kumar, V.;John, R. P. Polyhedron 2017,134, 11-21.
CrossRef - Khandani, M.; Sedaghat, T.; Erfani, N.; Haghshenas, M.R.; Khavasi, H.R. J. Mol. Struct.,2013,1037, 136.
CrossRef - Akbari, A.; Ghateazadeh, H.; Takjoo, R.; Nejad, B.S.; Mehrvar, M.; Mague, J.T. J. Mol. Struct.,2019,1181, 287.
CrossRef - Rama Jyothi, N.; Mohamed Farook, N.A. Chem. Sci. Trans., 2018, 7(4), 626.
- Rama Jyothi, N.; Mohamed Farook, N.A. Chem. Sci. Trans., 2018, 7(4), 634.

This work is licensed under a Creative Commons Attribution 4.0 International License.









