Investigation of Anticancer Agents from the Bark of Gyrinops Versteegii (Gilg.) Domke from Lombok Island
Surya Hadi1*, Dian Lestari1, Ni Komang Tri Dharmayani1, Baiq Desy Ratnasari2, M Ito3, I Yamada4 and Tri Mulyaningsih5
1Chemistry Department, The University of Mataram, Mataram, Indonesia.
2Departmen of Pharmacy, Stikes Kusuma Bangsa Mataram.
3Graduate School of Pharmaceutical Sciences, Kyoto University, Japan.
4Center for Southeast Asian Studies, Kyoto University, Japan.
55Biology Department, The University of Mataram,, Mataram, Indonesia.
Corresponding Author E-mail: sur_hadi88@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/360605
This study is part of a research collaboration between Kyoto University and The University of Mataram, aiming to identify and to utilize the agarwood plants, i.e., Gyrinops versteegii from Indonesia. The study, specifically, aims to discover anticancer agents from the bark of G. versteegii from Lombok Island of Indonesia. There were three provenances of G. versteegii observed, namely Soyun, Pantai, and Buaya. Based on the Brine Shrimp Lethality Test (BSLT), G. versteegii Buaya showed the highest cytotoxicity with LC50 45,94 µg/ml. Meanwhile, G. versteegii Soyun and Pantai have LC50 75.86 µg/mL and 56.36 µg/mL respectively. A phytochemical study showed that the methanol extract of G. versteegii Buaya containing compounds from the group of alkaloid, flavonoid, steroid, triterpenoid, and saponin. The methanol extracts fractionated using Vacuum Liquid Chromatography into 10 fractions (F1-F10) and then retested BSLT. Among the fractions, F2 showed the best potency as an anticancer with LC50 64,12 µg/mL. Based on the GC-MS analysis, the cytotoxicity from both methanol extract and F2 is predicted to be influenced by the same compounds, namely, 1,4-Benzenediol,2-methyl, Pyridoxylamine, 2,3-Dimethylhydroquionone, Tetramethyl-p-benzoquinone, and Benzofuran. Overall, the bark of G. versteegii from Lombok Island has great potency as an anticancer.
KEYWORDS:Agarwood; Anticancer; Gyrinops Versteegii; Lombok Island; Provenance
Download this article as:| Copy the following to cite this article: Hadi S, Lestari D, Dharmayani N. K. T, Ratnasari B. D, Ito M, Yamada I, Mulyaningsih T. Investigation of Anticancer Agents from the Bark of Gyrinops Versteegii (Gilg.) Domke from Lombok Island. Orient J Chem 2020;36(6). |
| Copy the following to cite this URL: Hadi S, Lestari D, Dharmayani N. K. T, Ratnasari B. D, Ito M, Yamada I, Mulyaningsih T. Investigation of Anticancer Agents from the Bark of Gyrinops Versteegii (Gilg.) Domke from Lombok Island. Orient J Chem 2020;36(6). Available from: https://bit.ly/3kXs1Wj |
Introduction
In an attempt to preserve the germplasm of agarwood, especially in the West Nusa Tenggara, a collection and an identification of agarwood plant is continuously conducted. Gyrinops versteegii(Gilg.) Domkeis one of the agarwood species found in Nusa Tenggara, specifically Lombok Island. This species is classified as superior agarwood that produces high-quality resin. As a result, the demand for G. versteegii is increasing by the year. Thus, exploration and plantation of this species are vigorously conducted, specifically in Lombok’s forest.1-3G. versteegii in West Lombokis found in five provenances, namely “G. versteegii Buaya”; “G. versteegii Soyun”; “G. versteegii Pantai”; “G. versteegii Madu”; and “G. versteegii Beringin”.2,4
The aromatic resin of the agarwood is the most attractive part of the plant. It is processed as fragrant oil to produce perfume and medicine. The other parts of the plant such as the leaves, the barks, the fruits, and the flowers, are not fully recovered industrially and scientifically. Nevertheless, they possess many valuable bioactivities to maintain health and to treat some ailments like cirrhosis, tumor, and cancer.4 However, a clinical study of those bioactivities has not been conducted widely, especially in Lombok Island. Therefore, the total utilization of agarwood parts, especially the barks, not only the resin part, is eagerly needed.
Hadi et al.,5pronounced the major compound of G. versteegiibark a flavonoid.Flavonoid is well known to possess ananti-cancer activity. However, the study has not been conducted widely. Generally, agarwood plants have good bioactivity as anti-cancer. Another agarwood species like Aquilaria malaccensis was reported to inhibit the proliferation of HCT116 with IC504 µg/mL.6 Besides, it also effective against lymphocytic leukemia cell P-388 with ED50 0,35 µg/mL.7 Further study found that the secondary metabolites, 2-(2-phenylethyl) chromones, that was isolated from agarwood was showing anti-cancer activity with IC50 14,6µg/mL against SGC-7901.8 Thus, theoretically, the species of Gyrinops has the same potential as the Aquilaria’s as an anti-cancer agent.
This research is designed to investigate the compound that is responsible for the anti-cancer in the bark of G. versteegii of Lombok Island from three provenances (G. versteegiiSoyun, G. versteegiiPantai and G. versteegiiBuaya) using Brine Shrimp Lethality Test (BSLT). Furthermore, the chemical compound in the barks was identified based onGas Chromatography-Mass Spectrometry (GC-MS).
Material and Methods
Preparation
The bark samples were obtained from three provenances of G. versteegii(Soyun, Pantai, and Buaya) from various places of Lombok Island, namely “Dusun Kerujuk, Pemenang Barat Village” and “the forest of Pusuk Lestari, Lembah Sari village”.
Maceration and Separation
500 gram of each samples (Soyun, Pantai, and Buaya) were dried then mashed finely into powder before macerated with methanol for 3×24 h. The crude extracts of each samples then tested using the Brine Shrimp Lethality Test (BSLT) to identify their toxicity level. The most toxic extract then chosen for the next step of separation using a vacuum liquid chromatography (VLC) technique. The stationary phase was silica gel GF60, while the mobile phase was n-heksane, DCM, methanol, etylacetate and aceton. The fractions that have the same retention factor (RF) in thin layer chromatography (KLT) were combined then were retested using BSLT to detect the most toxic fraction. Finally, the chemical compounds in the most poisonous fraction were identified applying gas chromatography-mass spectrometry (GC-MS).
Phytochemical test
Phytochemical test was conducted to analyze the compound group in the extracts qualitatively. The most toxic provenance and the combinations of the fractions from VLC was tested alkaloid, flavonoid, steroid, triterpenoid, and saponin using respective reagent.
Toxicity test using BSLT
The toxicity test was conducted using BSLT. First of all, stock solutions were made by adding 50 mg of samples into 5 mL of DMSO. The stock solutions then diluted into various concentrations, namely 10 ppm, 100 ppm, and 1000 ppm (Test Solutions). 5 mL of each test solutions were put into a tube, then added with ten (10) Artemia salina L. Larvae. The toxicity level was analyzed by counting larvae mortality after observed for 24 h. The mortality percentage was counted using Abbot formula.9
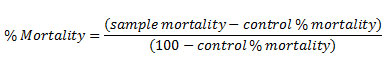
The mortality percentage then conversed into LC50 using statistical analysis (Probit and regression analysis).10
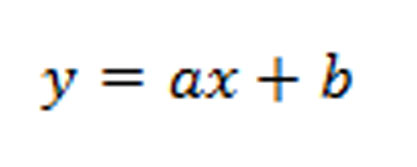
GC-MS Analysis
The GC-MS type is QP2010S SHIDMADZU. In the analysis, Helium (He) was used as the carrier gas. The column type was Rtx 5 semi-polar with 30 m long, 0.25 mm diameter, and 0.25µm film thickness. Helium was used as the gas carrier with flow rate 30 mL/minute. The molecular mass range of ions was identified at 35-500m/z. the temperature program: the injection temperature was set to 260oC with initial column started at 40oC for 5 min and programmed to increase 30oC per minute until it reached 260oC for 7 minutes.
Result
Toxicity test
Table 1: Toxicity result of the methanol extract of theG. versteegii bark from the three provenance
|
Provenance |
Concentration (ppm) |
Log concentration |
∑ larvae mortality rate after 24 h |
Mortality (%) |
LC50 ( µg/mL ) |
||
|
1 |
2 |
3 |
|||||
|
Soyun |
1000 |
3 |
10 |
10 |
10 |
100 |
75,86 |
|
100 |
2 |
3 |
3 |
2 |
27 |
||
|
10 |
1 |
1 |
2 |
1 |
13 |
||
|
Buaya |
1000 |
3 |
10 |
10 |
10 |
100 |
45,94 |
|
100 |
2 |
4 |
5 |
3 |
40 |
||
|
10 |
1 |
3 |
2 |
3 |
27 |
||
|
Pantai |
1000 |
3 |
10 |
10 |
10 |
100 |
56,36 |
|
100 |
2 |
4 |
5 |
4 |
43 |
||
|
10 |
1 |
2 |
0 |
3 |
17 |
||
|
Standard Sea Water |
– |
0 |
0 |
0 |
0 |
0 |
0 |
|
DMSO |
– |
0 |
0 |
0 |
0 |
0 |
0 |
Table 2: Toxicity result of the G. versteegii Buaya fractions
|
Fractions |
Concentration (ppm) |
Log concentration |
∑larvae mortality rate after 24 h |
Mortality (%) |
LC50 ( µg/mL) |
||
|
1 |
2 |
3 |
|||||
|
1,3,6,7,10 |
1000 |
3 |
10 |
10 |
10 |
100 |
489,89 |
|
100 |
2 |
0 |
0 |
0 |
0 |
||
|
10 |
1 |
0 |
0 |
0 |
0 |
||
|
2 |
1000 |
3 |
10 |
10 |
10 |
100 |
64,12 |
|
100 |
2 |
9 |
7 |
7 |
77 |
||
|
10 |
1 |
1 |
0 |
0 |
3 |
||
|
4 |
1000 |
3 |
10 |
10 |
10 |
100 |
116,81 |
|
100 |
2 |
0 |
2 |
1 |
10 |
||
|
10 |
1 |
1 |
1 |
0 |
7 |
||
|
5,9 |
1000 |
3 |
10 |
10 |
10 |
100 |
254,02 |
|
100 |
2 |
1 |
0 |
0 |
3 |
||
|
10 |
1 |
0 |
0 |
0 |
0 |
||
|
8 |
1000 |
3 |
10 |
10 |
10 |
100 |
165,20 |
|
100 |
2 |
1 |
0 |
0 |
3 |
||
|
10 |
1 |
1 |
0 |
0 |
3 |
||
Phytochemical test
Table 3: phytochemical study of the barks of G. versteegii Buaya
|
Compound Class |
Indication |
Result |
|
Alkaloid |
White Precipitation, the solution changes into green Brown precipitation, the solution changes into brownish oranges |
+ + |
|
Saponin |
Foam appear for ± 10 minutes |
+ |
|
Flavonoid |
The solution changes into blackish green |
+ |
|
Steroid |
Greenish blue ring appear in the solution |
+ |
|
Terpene |
The solution changes into brownish red |
+ |
GC-MS Analysis
The methanol extract has 57 compounds identified while the fraction-2 conveys 27 compounds.
 |
Figure 1: GC-MS chromatogram of the G. versteegii Buaya extract a. methanol extract b. fraction 2 |
Discussion
Toxicity test
The methanol extract of G. versteegii Soyun, G. versteegii Buaya, and G. versteegii Pantai was evaluated for the toxicity activity against Brine Shrimp Larvae. The macerate was diluted into 1000, 100, and 10 ppm and the result of the test is provided in Table 1.
Based on Table 1, the G. versteegii Buaya variety has shown the highest cytotoxicity with LC50 45,94 µg/m. A compound is classified as toxic if it has LC50< 1000 µg/mL according toMeyer et al.9. Thus, G. versteegii Buaya, as the most poisonousvariety, was chosen for the next steps that are phytochemical test and compound separation using liquid vacuum chromatography. The phytochemical test result is displayed in Table 3. Generally, the bark of G. versteegii Buaya is containing alkaloid, saponin, flavonoid, steroid, and terpene. Alkaloid and flavonoid have been known for their anti-cancer and anti-tumor properties.11Hence, a further separation of the methanol extract was conducted.
The separation of the G. versteegii Buaya extract with liquid vacuum chromatography yielding ten fractions (F1-F10) that were obtained by adding the solution polarity with the eluen of n-hexane: ethyl acetate. The ratio were 10:0 ; 9:1 ; 7:3 ; 6:4 ; 5:5 ; 3:7 ; 1:9 respectively. These fractions then retested their toxicity level using BSLT and the result is shown in Table 2.
Based on Tabel 2, F2 has the highest toxicity activity against the Artemia salina L. larvae with LC50 64.12 µg/mL. According to Hamidi et al.,10this result is classified as forceful toxicity. If the LC50 is around 500-100 µg/mL, it is classified as weak, while 100-500 µg/mL is moderate, and 0-100 µg/mLis strong. Nevertheless, the toxicity of F2 is weaker compared to the crude methanol extract of the G. versteegii Buaya,which have LC50 45.94 µg/mL. The strong-toxicity activity is associated with the number of metabolites in the extract. Overall, these results reveal a potency of the G. versteegii “Buaya” as a new anti-cancer agent. The chemical compound in the methanol extract and the F2 was identified based on GC-MS.
GC-MS Analysis
According to the literature review, overall, five anti-cancer compounds are detected in both methanol extract and F2. Those compounds are 1,4-Benzenediol,2-methyl (1), Pyridoxylamine (2), 2,3-Dimethylhydroquionone (3), Tetramethyl-p-benzoquinone (4), and Benzofuran (5). The abundance percentage in the extracts is displayed in Table 4.
Table 4: Anticancer compounds in the methanol extract and the fraction 2 of G. versteegii Buaya
|
No |
Compound |
% Area in MeOH extract |
% Area in Fraction 2 |
|
1 |
1,4-Benzenediol,2-methyl (1) |
1.23 |
0.68 |
|
2 |
Pyridoxylamine (2) |
1.28 |
5.64 |
|
3 |
2,3-Dimethylhydroquionone (3) |
2.37 |
3.79 |
|
4 |
Tetramethyl-p-benzoquinone (4) |
3.67 |
72.25 |
|
5 |
Benzofuran (5) |
3.30 |
1.42 |
Tetramethyl-p-benzoquinone (4) or known as duroquinone is a part of benzoquinone compound. It is detected as the most abundance compound in both methanol extract and F2. Benzoquinone has been proved to have potential in treating breast cancer. The compound has effectively inhibiting the proliferation of estrogen receptor-positive MCF-7 cells through the NF-κB pathway via estrogen receptor signaling.12 Other quinone compounds also detected in the agarwood barks extracts as hydroquinone group, which are 1,4-Benzenediol,2-methyl (1), and 2,3-Dimethylhydroquionone (3). They are reported to successfully induce the death cell of A431 epidermal cell lines, SYF (Src, Yes, and Fyn), B16F10, and MDA-MB-231-cells that are implicated as key regulators of ligand-induced cellular responses including proliferation, survival, adhesion, and migration. Furthermore, the process of the cell cancer inducement is rated to be safe and efficient without adverse effect.13
Pyridoxylamine (2), commercially known as vitamin B6, is a primary metabolite that is important for metabolism. It has a great record of its anti-cancer activity. It is reported that pyridoxylamine has suppressed cell proliferation of B16 and B16F10 murine melanoma cells and M21-HPB human melanoma cells.14Benzofuran (5) and its derivatives like 5-Methyl-benzofurazan (6) have reported to effectively kill cancer cells line like lipoma, Hella, ovarian cancer, and leukemia cells by inhibiting the cell proliferation. In addition, benzofuran and its derivative also have antitumor activity against MCF-7 dan PC-3.15-19
 |
Figure 2: Anticancer compounds in the methanol extract and theF2 of G. versteegii Buaya |
Other compounds that are detected in the methanol extract of G. versteegii Buaya are α-Caryophyllene (6) β-Santalol (7). α-Caryophyllene (6), known as humulene, is sesquiterpenes that is found in the essential oil. This compound is one of the responsible compounds for the aromatic scent. Furthermore, this compound is also known for its anti-cancer activity. According to Ambroz et al.,20, 21 Humulene is an effective treatment for intestinal, ovarian, and lymphoblast cancer by inhibiting the proliferation process and oxidating effect.
β-Santalol is one of the substantial compounds in sandalwood essential oil (SEO). An exposure of SEO (2–8 μg/mL for 24 h) is capable of breaking the single- and double-strand DNA in the MCF-7 cells, a human breast cell line. This genotoxic activity is contributed by the presence of β-Santalol, along with α-Santalol.22
 |
Figure 3: Other compounds that are detected in the barks of methanol extract of G. versteegii Buaya |
Conclusion
Among G. versteegii provenances that are found in the Lombok Island, G. versteegii Buaya has the most toxic effect on the brine shrimp larvae that consequently makes it as the most potential provenance to be developed as anticancer drugs. Specifically, the methanol extract was more active in inhibiting larvae reproduction. The cytotoxicity from both methanol extract and F2 is predicted to be influenced by the same compounds, namely, 1,4-Benzenediol,2-methyl, Pyridoxylamine, 2,3-Dimethylhydroquionone, Tetramethyl-p-benzoquinone, and Benzofuran. Moreover, the methanol extract has two additional compounds (α-Caryophyllene and β-Santalol) that strengthen its cytotoxic activity.
Acknowledgement
We would like to give sincere gratitude to MENRISTEK (Ministry of Research and Technology) of Indonesia for funding this research.
Conflict of Interest
The authors declare that this research has no conflict of interest.
References
- Mulyaningsih, T.; Yamada, I.Natural resource management and socio-economic transformation under the decentralization in Indonesia: Toward Sulawesi area studies. 2007, 365-372
- Mulyaningsih, T.; Marsono, D.; Sumardi, S.; Yamada, I.Jurnal Penelitian Hutan dan Konservasi Alam. 2017,14 (1), 57-67
CrossRef - Mulyaningsih, T.; Marsono, D.; Sumardi; Yamada, I.Ecology, Environment and Conservation Paper.2017,23,723-729
- Setyaningrum, H. D.; Saparinto, C.Penebar Swadaya Grup. 2014
- Hadi, S.; Muliasari, H.; Sukma, N. S.; Ratnaningsih, P. E. W.Proceeding of the 2th International Seminar on Chemistry, Jatinangor.2011, 79-82
- Ibrahim, A. H.; Al-Rawi, S. S.; Majid, A. M. S. A.; Rahman, N. N. A.; Abo-Salah, K. M.; Ab Kadir, M. O. Procedia Food Science. 2011,1, 1953-1959
CrossRef - Gunasekera, S. P.; Faircloth, G. T. The Journal of Organic Chemistry. 1990,55 (25), 6223-6225
CrossRef - Liu, J.; Wu, J.; Zhao, Y. X.; Deng, Y. Y.; Mei, W. L.; Dai, H. F. Chinese Chemical Letters. 2008,19 (8), 934-936
CrossRef - Meyer, B. N.; Ferrigni, N. R.; Putnam, J. E.; Jacobsen, L. B.; Nichols, D. E. j.; McLaughlin, J. L.Planta medica. 1982,45 (05), 31-34
CrossRef - Hamidi, M. R.; Jovanova, B.; Panovska, T. K.Maced pharm bull. 2014,60 (1), 9-18
CrossRef - Bhavana, V.; Sudharshan, S. J. S.; Madhu, D. Anticancer plants: clinical trials and nanotechnology, Springer. 2017, 51-104
CrossRef - Zheng, L.; Cai, Y.; Zhou, L.; Huang, P.; Ren, X.; Zuo, A.; Meng, X.; Xu, M.; Liao, X. International Journal of Molecular Medicine. 2017,39 (1), 39-46
CrossRef - Byeon, S. E.; Yi, Y.-S.; Lee, J.; Yang, W. S.; Kim, J. H.; Kim, J.; Hong, S.; Kim, J.-H.; Cho, J. Y. International journal of molecular sciences. 2018,19 (3), 903
CrossRef - Matsuo, T.; Sadzuka, Y. Anticancer research. 2019,39 (7), 3429-3432
- Napiórkowska, M.; Cieślak, M.; Kaźmierczak-Barańska, J.; Królewska-Golińska, K.; Nawrot, B. Molecules. 2019,24 (8), 1529
CrossRef - Santha, S.; Dwivedi, C. Anticancer research.2015,35 (6), 3137-3145
- Shah, C. P.; Kharkar, P. S.Journal of enzyme inhibition and medicinal chemistry.2018,33 (1), 972-977
CrossRef - Xu, J.-P.CRC Press. 2016
- Coşkun, D.; Tekin, S.; Sandal, S.; Coşkun, M. F. Journal of Chemistry.2016
CrossRef - Ambrož, M.; Boušová, I.; Skarka, A.; Hanušová, V.; Králová, V.; Matoušková, P.; Szotáková, B.; Skálová, L. Molecules.2015,20 (8), 15343-15358
- Ambrož, M.; Matoušková, P.; Skarka, A.; Zajdlová, M.; Žáková, K.; Skálová, L. Molecules.2017,22 (6), 1021
CrossRef - Ortiz, C.; Morales, L.; Sastre, M.; Haskins, W. E.; Matta, J. Evidence-Based Complementary and Alternative Medicine.2016
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









