Enhance the Stability of Traditional Concentrated Yoghurt Using Natural Antioxidants Additives
Ziad Ayyad1 , Muhannad Qurie2, Amal Odeh Natshe2
, Muhannad Qurie2, Amal Odeh Natshe2 , Saleh Sawalha1
, Saleh Sawalha1 and Fuad Al-Rimawi3*
and Fuad Al-Rimawi3*
1Department of Food Engineering, Faculty of Engineering, Al-Quds University, Palestine.
2Department of Environment and Earth Sciences, Faculty of science and technology, Al-Quds University, Palestine
3Department of Chemistry and Industrial Chemistry, Faculty of Science and Technology, Al-Quds University, Palestine.
Corresponding Author E-mail: falrimawi@staff.alquds.edu
DOI : http://dx.doi.org/10.13005/ojc/360512
Article Received on : 07-08-2020
Article Accepted on : 16-09-2020
The stability of virgin olive oil (VOO) used as a packing medium for traditional concentrated Yoghurt decreased during time and the product could deteriorate during the storage time. In this investigation, different natural additives such as dried Arum Palaestinum leaves (AP), Tomato Peel (TP) and Chili Pepper (CP) have been used to enhance the quality and stability of packing medium VOO for traditional canned concentrated Yoghurt balls. Parts VOO samples added with natural additives were stored as packing medium for traditional canned concentrated Yoghurt balls. Other part was stored without concentrated Yoghurt in the same storage conditions. All samples were analyzed for their initial quality indexes and during the storage period of six months. At the end of storage, results revealed that the % acidity for all VOO samples used as a packing medium showed a higher value than the samples stored without concentrated Yoghurt balls. On the other side, peroxide values for all stored samples of both parts were less than the control sample without additives. Extinction coefficients (K232, K270) for VOO samples with the natural additives showed increased trend during the storage time, but it didn't exceed the accepted limit for VOO. Total phenol content for all samples were gradually decreased during storage period, whereas samples with the natural additives showed higher values than the controls. All the natural additives (CP, TP, AP) showed a positive trend in enhancing and improving the different VOO quality indexes in our study in particular those samples added with CP during the storage time.
KEYWORDS:Arum Palaestinum; Chili Pepper; Natural Additives; Quality Indexes; Storage Stability; Tomato Peel; Virgin Olive Oil Traditional Concentrated Yoghurt
Download this article as:| Copy the following to cite this article: Ayyad Z, Qurie M, Natshe A. O, Sawalha S, Al-Rimawi F. Enhance the Stability of Traditional Concentrated Yoghurt Using Natural Antioxidants Additives. Orient J Chem 2020;36(5). |
| Copy the following to cite this URL: Ayyad Z, Qurie M, Natshe A. O, Sawalha S, Al-Rimawi F. Enhance the Stability of Traditional Concentrated Yoghurt Using Natural Antioxidants Additives. Orient J Chem 2020;36(5). Available from: https://bit.ly/2FVrEgJ |
Introduction
Virgin olive oil composed mainly of oleic acid and a balanced amount of polyunsaturated fatty acids, which qualifies it as natural antioxidants. In addition to its contribution to the prevention of several human diseases, as cardiovascular diseases, VOO found to have a major contribution in cancer disease prevention [1]. Secoiridoid molecules are the direct response on the sensory gustative properties of VOOs; pungency, bitterness, and other sensory properties [2]. Additionally, VOO contains more than 230 chemical compounds as minor components that composed around 2% of its weight [3].
Oxidative stability is considered useful indicator for the estimation of the potential shelf life of VOO due to the high stability of VOO under normal storage conditions (12 to more than 18 months) [4]. The amount of antioxidants is a key indicator of the quality and stability of VOO, including carotene, phenols, and hydrophilic phenols. Hydrophilic phenols are the most abundant natural antioxidants of VOO [5]. Phenolic compounds concentration ranges from 1.0 to 3.0% (weight / weight) for olive fruits [3]. The antioxidant content, particularly polyphenols give VOO high resistant to oxidation compared with other edible oils [6-8]. Natural sources of antioxidants such as tocopherols, ascorbic acid, erythorbic acid or their salts or derivatives have found extensive applications in the food industry [9]. They were characterized by low cost, availability, and recycling without the release of toxic compounds. Other advantages made them environmentally friendly and can be used as natural additives in various foods [10] in order to avoid the adverse health effects of synthetic antioxidants [11]. The use of different plants in Palestine either in traditional medicine or as edible plants is justified since they are promising sources of natural antioxidants [12]. Natural phytochemicals derived from fruits, vegetables, and herbs possess a wide range of biological effects including antioxidant, antimicrobial and anti-inflammatory actions. Addition of different spices, herbs, and olive oil is a traditional practice in Mediterranean gastronomy to enhance the aroma and taste of food [12]. One of the well-known plant used as traditional food and possess natural antioxidants found in Palestinian nature is the Arum Palaestinum (AP). Such plant (locally known as “Luof”).contains different active micro-components such as polyphenols, alkaloids, flavone C-glycosides, flavonols, flavones, proanthocyanidins, and polyhydroxy alkaloids. The leaves are considered one of the most edible wild plants in Palestine and one of the medicinal plant utilized for treating various diseases especially cancer [13]. Arum Palaestinum is commonly preserved by air-drying and then stored refrigerated or frozen and might be used in many spices mixes [13]. Other natural plants or plant parts that are rich in antioxidants are tomato peels with its content of lutein, lycopene, and b-carotene. [14], and chili pepper (CP). CP is a rich source of vitamins C, E and carotenoids [15]. Moreover, CP is considered a good source of flavonoids such as quercetin and luteolin [16]. CP could be added to olive oil to prevent it from oxidations [17]. On the other hand, CP play clear rule in exhibiting the strongest ferrous chelating activity and scavenging activity against free radicals [16].
The content of phenolic compounds and ascorbic acids, β-carotene, α-tocopherol, and lycopene, play an important role in increasing total antioxidant activity of such natural antioxidants [18].
This investigation is based on the use of a traditional fermented dairy product (Concentrated Yoghurt). Concentrated Yoghurt is a semisolid fermented dairy food produced by removing part of the whey from yogurt to reach total solid levels between 23 and 25 g/100 g. concentrated Yoghurt balls are immersed in VOO olive oil as a packing medium to preserve it and to increase its shelf life at the same time. It may be kept in the refrigerator for up to 2 months. Dairy products as many other food products are perishable by nature and require protection from spoilage during their preparation, storage and distribution to give them desired shelf life [19]. The objectives of this work is therefore to study the (Arum Palaestinum, Tomato peel, and Chili Pepper) on the quality of VOO which is used as packing medium for Concentrated Yoghurt balls.
Materials and Methods
Samples
VOO of the cultivar “Nabali Baladi” from Salfeet region- West Bank was extracted in November 2016. Leaves of Arum palaestinum plant were collected from the mountainous area near Ramallah /Palestine. Tomato and green fresh pepper were obtained in the same period from markets. Leaves of Arum Palestinian, tomato peel and green pepper were dried at room temperature. The dried natural additives were then packaged and stored at 20 ± 2°C in the dark until used. Traditional concentrated Yoghurt balls were prepared from fresh and pasteurized milk.
Samples Preparation
The VOO was enriched with natural additives according to Table 1 and divided into two parts. The first part was stored in a hermetically sealed glass bottle in dark at room temperature. The other part was added as packing medium to traditional concentrated Yoghurt balls in a hermetically sealed glass jars. Control samples were prepared for each part without any additives. All samples were stored at room temperature (20±2 oC) in dark for a period of 6 months. Olive oil samples were analyzed initially at time zero and each respective time of analysis during the period of storage for their free acidity, peroxide value, UV absorption coefficients, and total phenols. Each sample was obtained from separate sealed bottles and jars and was analyzed in triplicates.
Table 1: Distribution of natural additives in VOO samples.
|
Natural Additives |
Concentration% |
(g) of natural additives in 85g of VOO samples |
(g) of natural additives in 185g of VOO & 255g Concentrated Yoghurt |
|
Control |
———- |
———- |
|
|
Arum Palaestinum |
2% |
1.7 |
3.7 |
|
5% |
4.25 |
9.25 |
|
|
10% |
8.5 |
18.5 |
|
|
Tomato Peel |
2% |
1.7 |
3.3 |
|
5% |
4.25 |
9.25 |
|
|
10% |
8.5 |
18.5 |
|
|
Chili Pepper |
1% |
0.85 |
1.85 |
|
2% |
1.7 |
3.7 |
|
|
3% |
2.55 |
5.55 |
Chemical Quality Indexes
Acidity and peroxide values were expressed as g oleic acid per 100 g oil and as milliequivalent O2 per Kg oil, respectively, both indicators were analyzed according to the methods described in AOAC, 2000. Extinction coefficients K232 and K270 were performed according to the methods described in IOOC. Total polar phenols were extracted following the procedure by Georgios et al and the results were expressed as mg gallic acid per Kg oil.
Statistical Analysis
All chemical analyses were carried out in triplicate. The software XLSTAT 7.5.2 version (Addinsoft, USA) was used to elaborate the data by analysis of variance (ANOVA, Fisher LSD, p < 0.05). Significant differences (at p-level < 0.05) among medians at different storage time was used. The same letters (a-d) represent no significant differences throughout the storage time, within the same sample (P < .05).
Chemicals
All chemicals used in this study are of analytical grade and purchased from Fluka (Buchs, Switzerland) or Sigma-Aldrich (Steinheim, Germany). The list of, chemicals include: Acetic acid, Chloroform Potassium Iodide, Sodium Thiosulfate, Starch Ethanol 95%, Potassium Hydroxide, Phenol Phethalein, Hydrochloric Acid, Cyclohexane, Sodium Carbonate, Folin-Ciocalteu Reagent).
Results and Discussion
VOO Enriched with Natural Additives
Acidity Percent
Results (figures 1-3) showed significant increase in acidity % of VOO for the control samples compared with those with AP,TP and CP during six months of storage. On the other hand, the samples enriched with CP contain less free fatty acid percent than other samples. This could be explained by the action of various antioxidants contained in, such as polyphenols and alkaloids with flavone glycosides, flavonols, flavones, proanthocyanidins, and polyhydroxy alkaloids as main classes in (AP) [20]. These results are in agreements with [21] who found that enrichment of VOO with carotenoids and lycopene from (TP), caused decrease in acidity during storage time compared with the control sample. Besides, Borel and his coworkers [22] explained this decline in acidity by the solubility of carotenoids from (TP) in the olive oil. In addition, the lower values of acidity in VOO samples contained CP reflects improvement effects of CP on the oxidative stability of VOO (Fig 3). In VOO samples with (AP) and (TP), 2% concentration was more efficient than the higher 5% and 10% in keeping stable oil with acceptable acidity %. Nevertheless, all stored control samples have remained within the limits of EU regulations (EU Re.1348/2013) at the end of the storage period.
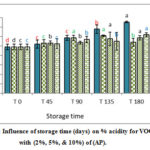 |
Figure 1: Influence of storage time (days) on % acidity for VOO samples with (2%, 5%, & 10%) of (AP). |
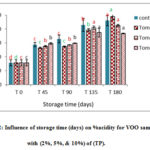 |
Figure 2: Influence of storage time (days) on %acidity for VOO samples with (2%, 5%, & 10%) of (TP). |
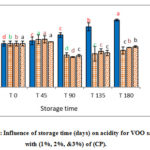 |
Figure 3: Influence of storage time (days) on acidity for VOO samples with (1%, 2%, &3%) of (CP). |
Peroxide Value for VOO
P.V results (Figures 4-6) indicated significant increase in P.V values during storage. However, control sample showed generally significantly higher increase in PV with respect to natural additives enriched samples throughout the storage time.
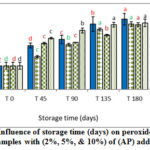 |
Figure. 4: Influence of storage time (days) on peroxide value for VOO samples with (2%, 5%, & 10%) of (AP) additives. |
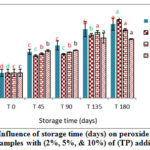 |
Figure 5: Influence of storage time (days) on peroxide value for VOO samples with (2%, 5%, & 10%) of (TP) additives. |
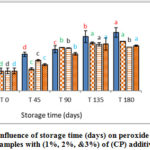 |
Figure 6: Influence of storage time (days) on peroxide value for VOO samples with (1%, 2%, &3%) of (CP) additives. |
These results emphasize the positive effect of natural additives (CP, AP and TP) on the oxidative stability of VOO. The results also indicated that higher concentrations of natural antioxidant have better effects on oxidative stability than lower concentrations. These results were in agreement with [23-24].
Extinction Coefficients ( K232, K270 )
A significant increase in K232 and K270 values were observed for all samples with different concentrations of natural additives in addition to the control sample (Table 2 and 3). However, all the samples remained within the accepted limits by EEC Reg. 2568/1991 and the following amendments for VOO category.
Table 2: Influence of natural additives on the extinction coefficients (K232) value indicators for the pure VOO during 6 months of storage.
|
sample |
Type |
K232±S.D at T0 |
K232±S.D at T45 |
K232±S.D at T90 |
K232±S.D at T135 |
K232±S.D at T180 |
|
control |
V |
2.21±0.04c |
2.31±0.02c |
2.397±0.17b |
2.52±0.04a |
2.58±0.14a |
|
V-O.O |
C1(2%) |
2.21±0.04b |
2.31±0.14ab |
2.09±0.03c |
2.35±0.06a |
1.45±0.05d |
|
with |
C2(5%) |
2.21±0.04c |
2.36±0.24b |
2.11±0.18c |
2.33±0.08a |
1.48±0.06d |
|
Arum |
C3(10%) |
2.21±0.04b |
2.33±0.01a |
2.17±0.01c |
2.29±0.17ab |
1.5±0.06d |
|
V-O.O |
C1(2%) |
2.21±0.04c |
2.42±0.14c |
2.36±0.08b |
2.41±0.0a |
1.88±0.08d |
|
with |
C2(5%) |
2.21±0.04b |
2.48±0.09bc |
2.30±0.22b |
2.50±0.11a |
1.98±0.04c |
|
Tomato |
C3(10%) |
2.21±0.04c |
2.48±0.05b |
2.30±0.28bc |
2.48±0.11a |
01.81±0.11d |
|
V-O.O |
C1(1%) |
2.21±0.04c |
2.5±0.21ab |
2.391±0.03b |
2.46±0.0a |
1.30±0.16d |
|
with |
C2(2%) |
2.21±0.04bc |
2.47±0.17b |
2.41±0.01a |
2.49±0.11a |
1.36±0.03c |
|
Chili |
C3(3%) |
2.21±0.04c |
2.59±0.07a |
2.37±0.14b |
2.51±0.11a |
1.89±0.17d |
Table 3: Influence of natural additives on the extinction coefficients (K270) value indicators for the pure VOO during 6 months of storage.
|
sample |
Type |
K232±S.D at T0 |
K232±S.D at T45 |
K232±S.D at T90 |
K232±S.D at T135 |
K232±S.D at T180 |
|
control |
V |
2.355±0.14e |
2.63±0.13c |
2.75±0.13b |
2.59±0.18d |
2.87±0.05a |
|
V-O.O |
C1(2%) |
2.355±0.14d |
2.478±0.19c |
2.55±0.19b |
2.17±0.06e |
2.68±0.16a |
|
with |
C2(5%) |
2.355±0.14c |
2.522±0.12b |
2.61±0.12ab |
2.18±0.07d |
2.66±0.12a |
|
Arum |
C3(10%) |
2.355±0.14c |
2.538±0.14b |
2.56±0.14b |
2.19±0.23d |
2.63±0.19a |
|
V-O.O |
C1(2%) |
2.355±0.14d |
2.51±0.02b |
2.17±0.02e |
2.45±0.17c |
2.73±0.11a |
|
with |
C2(5%) |
2.355±0.14d |
2.521±0.02b |
2.18±0.02e |
2.46±0.09c |
2.680±0.06a |
|
Tomato |
C3(10%) |
2.355±0.14d |
2.55±0.04b |
2.189±0.04e |
2.508±0.05bc |
2.76±0.24a |
|
V-O.O |
C1(1%) |
2.355±0.14d |
2.47±0.01c |
2.70±0.01a |
1.92±0.14e |
2.54±0.08b |
|
with |
C2(2%) |
2.355±0.14c |
2.49±0.01b |
2.71±0.01a |
2.502±0.03b |
2.50±0.29b |
|
Chili |
C3(3%) |
2.355±0.14c |
2.56±0.03b |
2.74±0.03a |
2.07±0.13d |
2.56±0.21b |
The increase in K232 values is caused by the formation of primary oxidation products (hydro-peroxide) while that for K270 could be attributed to the accumulation of secondary oxidation products [25]. Such secondary products include aldehyde, ketones and others trienes containing product [26].
Total Phenolic Content for VOO
Figures 7- 9 represent the behavior of total phenolic compounds contents throughout the storage period for VOO samples added with the three natural antioxidants in comparison with the control sample. The results showed that total polar phenolic content for all samples decreased during storage, where samples with the natural additives had lower values at the end of storage period compared with control VOO sample. These results were in agreement with Montesano and his coauthors [24].
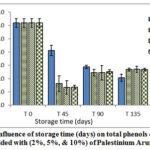 |
Figure 7: Influence of storage time (days) on total phenols content for VOO added with (2%, 5%, & 10%) of Palestinium Arum (AP). |
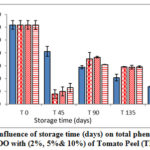 |
Figure 8: Influence of storage time (days) on total phenols content for VOO with (2%, 5%& 10%) of Tomato Peel (TP). |
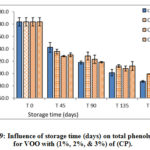 |
Figure 9: Influence of storage time (days) on total phenols content for VOO with (1%, 2%, & 3%) of (CP). |
The results of this research highlighted the protective effect of the natural additive on the phenolic compounds and the stability of VOO.
VOO as a Packing Medium for Traditional Concentrated Yoghurt Acidity Percentage
A significant increase in the free acidity values up to the end of storage period was observed while the control sample showed less increasing trend compared with those packed the concentrated Yoghurt balls in sealed glass jars (Figures 10-12). In addition, samples for packing VOO with 1%, 2%, & 3% of dried (AP), (TP), and (CP) were used. In addition, packing VOO samples showed similar trend as the samples stored without food matrix (Figure 1-3). The results showed also that lower concentration of the natural additives (1 %, 2%) used as packing medium showed lower acidity % than those added with 3%. However, all samples added with natural additives showed less acidity % than control samples at the end of storage period which indicate the positive effect of such additives on retarding hydrolytic degradation of VOO.
 |
Figure 10: Influence of storage time (days) on acidity for (VOO) samples with (2%, 5%, & 10%) of (AP) used as a packing medium for concentrated Yoghurt balls. |
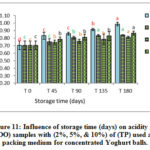 |
Figure 11: Influence of storage time (days) on acidity for (VOO) samples with (2%, 5%, & 10%) of (TP) used as a packing medium for concentrated Yoghurt balls. |
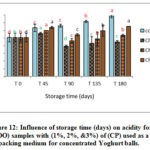 |
Figure 12: Influence of storage time (days) on acidity for (VOO) samples with (1%, 2%, &3%) of (CP) used as a packing medium for concentrated Yoghurt balls. |
Comparing the samples of VOO stored with and without concentrated Yoghurt balls, results showed that VOO samples used as packing medium had slightly higher acidity value, which could be explained by the humidity in concentrated Yoghurt (food matrix) which in turn increases the rate of the hydrolysis [3].
Peroxide Value
Figures 13-15 showed the results for VOO samples used as a packing medium for traditional concentrated Yoghurt with the natural additives (AP, TP, and CP). Results showed significant increase in PV for all samples during storage with respect to the initial values. Moreover, it was clear that peroxide values for VOO samples with natural additives had lower values than the control sample. The results emphasize the positive effect of these natural additives in enhancing the oxidative stability and quality of VOO used as a packing medium for traditional concentrated Yoghurt.
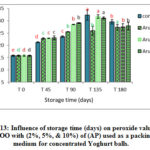 |
Figure 13: Influence of storage time (days) on peroxide value for VOO with (2%, 5%, & 10%) of (AP) used as a packing medium for concentrated Yoghurt balls. |
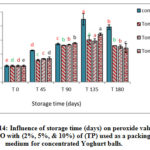 |
Figure 14: Influence of storage time (days) on peroxide value for VOO with (2%, 5%, & 10%) of (TP) used as a packing medium for concentrated Yoghurt balls. Click here to View figure |
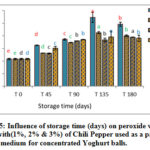 |
Figure 15: Influence of storage time (days) on peroxide value for VOO with(1%, 2% & 3%) of Chili Pepper used as a packing medium for concentrated Yoghurt balls. |
These results were in accordance with Montesano et al, 2006 who reported that VOO samples with lycopene showed PV remarkably lower than the reference sample. In addition, Caporaso and his group also reported that capsaicinoids and aroma compounds found in pepper had a high antioxidant capacity, that cause a decrease in (PV) and other oxidation parameters during VOO storage [12].
By comparing the activity of the different natural additives in view of peroxide value, results showed that the lowest recorded values of PV were for (TP) followed by (CP), then (AP) respectively.
Extinction Coefficients (K232, K270) for VOO with natural additives used as a packing medium for traditional concentrated Yoghurt
Results showed in Tables 4 and 5 indicated that K232 and K270 values exceeded the accepted limit by EEC Reg. 2568/1991 after 45 days of storage for all stored samples. In this regard, the sample with Chili pepper showed higher stability than other samples, where these results agreed with Caporaso et al., 2013. However, the increase in oxidation rate during storage of VOO used as a packing medium for concentrated Yoghurt might result from the presence of high humidity in concentrated Yoghurt. Besides, the presence of water in contact with oil considered responsible for the oxidation of double bonds in fatty acids, leading to an extreme increase in K232 and K270.
Table 4: Influence of natural antioxidants additives on the extinction coefficients (K232) Value Indicators for the pure VOO used as a packing medium for traditional concentrated Yoghurt during 6 months of storage.
|
sample |
Type |
K270±S.D at T0 |
K270±S.D at T45 |
K270±S.D at T90 |
K270±S.D at T135 |
K270±S.D at T180 |
|
control |
V |
0.195±0.04d |
0.234±0.04c |
0.237±0.04c |
0.247±0.04b |
0.277±0.01a |
|
V-O.O |
C1(2%) |
0.195±0.04d |
0.224±0.06c |
0.238±0.03b |
0.234±0.0b |
0.245±0.01a |
|
with |
C2(5%) |
0.195±0.04e |
0.226±0.05d |
0.231±0.05c |
0.242±0.02b |
0.249±0.03a |
|
Arum |
C3(10%) |
0.195±0.04e |
0.2252±0.01d |
0.239±0.01c |
0.246±0.03b |
0.251±0.04a |
|
V-O.O |
C1(2%) |
0.195±0.04d |
0.224±0.03c |
0.237±0.01b |
0.250±0.02a |
0.256±0.01a |
|
with |
C2(5%) |
0.195±0.04d |
0.229±0.02c |
0.233±0.04b |
0.251±0.07a |
0.253±0.05a |
|
Tomato |
C3(10%) |
0.195±0.04c |
0.23±0.04b |
0.235±0.04b |
0.253±0.02a |
0.255±0.01a |
|
V-O.O |
C1(1%) |
0.195±0.04c |
0.238±0.07b |
0.234±0.01b |
0.247±0.06a |
0.248±0.04a |
|
with |
C2(2%) |
0.195±0.04c |
0.237±0.05b |
0.233±0.0b |
0.243±0.01a |
0.244±0.0a |
|
Chili |
C3(3%) |
0.195±0.04d |
0.239±0.02c |
0.236±0.02c |
0.254±0.01a |
0.247±0.03b |
Table 5: Influence of natural antioxidants additives on the extinction coefficients (K270) value Indicators for the pure VOO used as a packing medium for traditional concentrated Yoghurt during 6 months of storage.
|
sample |
Type |
K270±S.D at T0 |
K270±S.D at T45 |
K270±S.D at T90 |
K270±S.D at T135 |
K270±S.D at T180 |
|
control |
0.245±0.01c |
0.268±0.05bc |
0.357±0.03b |
0.45±0.04ab |
0.480±0.01a |
|
|
VOO |
C1(2%) |
0.245±0.01c |
0.251±0.01bc |
0.274±0.02b |
|
0.313±0.01a |
|
with |
C2(5%) |
0.245±0.01d |
0.252±0.06c |
0.269±0.06b |
0.28±0.0b |
0.321±0.01a |
|
Arum |
C3(10%) |
0.245±0.01d |
0.251±0.05ac |
0.273±0.04b |
0.27±0.02b |
0.320±0.03a |
|
VOO |
C1(2%) |
0.245±0.01c |
0.258±0.0bc |
0.262±0.05b |
0.31±0.04b |
0.324±0.0a |
|
|
||||||
|
with |
C2(5%) |
0.245±0.01d |
0.261±0.05c |
0.263±0.06c |
0.31±0.02b |
0.321±0.03a |
|
Tomato |
C3(10%) |
0.245±0.01d |
0.265±0.01c |
0.266±0.04c |
0.31±0.03b |
0.326±0.01a |
|
VOO |
C1(1%) |
0.245±0.01c |
0.257±0.0b |
0.259±0.02b |
0.314±0.02a |
0.311±0.0a |
|
with |
C2(2%) |
0.245±0.01e |
0.259±0.02d |
0.265±0.02c |
0.322±0.01a |
0.315±0.02b |
|
Chili |
C3(3%) |
0.245±0.01e |
0.259±0.01d |
0.272±0.02c |
0.32±0.01a |
0.315±0.02b |
Total Phenolic Content
The results presented in Table 6 showed generally a significant and dramatic degradation of phenolic compounds in VOO samples, for all samples. These results indicated that an ultimate oxidation resistance was involved because of the presence of food (concentrated Yoghurt) in contact with VOO used in this study. A loss of about 50 % was recorded after the end of storage time.
Table 6:-Influence of natural antioxidants additives on the total phenols for of pure VOO and VOO that used as a packing medium covering the concentrated Yoghurt balls stored in hermetically sealed glass jars during 6 months of storage time.
|
sample |
media T.Ph±S.D at T0 |
media T.Ph ±S.D at T45 |
media T.Ph ±S.D at T90 |
media T.Ph ±S.D at T135 |
media T.Ph ±S.D at T180 |
|
|
VOO withnatural additives |
control |
183.74±6.76a |
172.34±7.77b |
117.85±2.49c |
101.45±5.26d |
87.28±1.50e |
|
C1 /A |
183.74±6.76a |
92.46±11.49c |
109.05±4.98b |
113.70±2.26b |
94.95±1.88c |
|
|
C2/A |
183.74±6.76a |
86.73±10.14c |
108.80±11.02b |
114.23±4.51b |
95.48±2.63c |
|
|
C3/A |
183.74±6.76a |
87.21±2.70d |
110.56±2.84b |
113.70±2.26b |
94.68±3.01c |
|
|
C1 /T |
183.74±6.76a |
75.74±6.76e |
130.67±9.95b |
118.22±0.38c |
97.61±1.13d |
|
|
C2/T |
183.74±6.76a |
79.80±7.10e |
133.43±1.07b |
117.95±3.01c |
99.4634±1.50d |
|
|
C3/T |
183.74±6.76a |
85.77±6.76d |
121.87±0.36b |
118.48±1.50b |
99.47±3.01c |
|
|
C1/Ch |
183.74±6.76a |
135.568±3.72c |
128.60±6.40b |
112.11±2.26b |
99.27±0.30c |
|
|
C2/Ch |
183.74±6.76a |
128.23±1.01b |
122.85±6.75b |
108.12±4.14c |
95.05±0.0d |
|
|
C3/Ch |
183.74±6.76a |
130.19±3.04b |
118.35±1.07c |
112.12±7.14c |
95.40±0.49d |
|
|
VOO withnatural additives for packing Labneh |
control |
154.59±8.79a |
119.76±2.70b |
100.756±8.89c |
83.929±3.76d |
75.42264±1.50e |
|
C1 /A |
154.59±8.79a |
129.411235±5.41d |
89.41±6.75b |
85.58±1.50d |
100.53±2.26b |
|
|
C2/A |
154.59±8.79a |
130.17±0.71b |
87.0183±0.71b |
83.797865±6.77d |
100.80±1.13b |
|
|
C3/A |
154.59±8.79a |
132.18±4.27b |
89.41059.18±4.27b |
84.5958±1.88d |
101.60±3.01b |
|
|
C1 /T |
154.59±8.79a |
65.70±2.03e |
97.49±7.11c |
73.56±0.38d |
105.58±4.89b |
|
|
C2/T |
154.59±8.79a |
67.14±2.70c |
101.01±2.84b |
74.36±8.27c |
99.20±4.89b |
|
|
C3/T |
154.59±8.79a |
67.14±4.73c |
101.76±11.73b |
74.95±2.18c |
100.53±3.76b |
|
|
C1/Ch |
154.59±8.79a |
63.79±3.38d |
103.52±1.42b |
78.88±4.14c |
106.65±4.14b |
|
|
C2/Ch |
154.59±8.79a |
66.66±7.43d |
108.05±10.66b |
79.14±3.76c |
101.60±0.75b |
|
|
C3/Ch |
154.59±8.79a |
68.33±2.37d |
110.31±3.91b |
80.47±5.64c |
103.72±2.26b |
|
The results (Table 6) for VOO with AP samples showed more stability in total phenol during storage, and it had higher values at the end of storage than the control samples. Moreover, results showed that phenolic substances seem to be involved more than other antioxidants in the protection of the oil from oxidation during storage [27]. The tomato peel and chili pepper additives, as shown in table 6, showed an increase in total phenols after 45 days of storage as a result of degradation of complex phenolic compounds [7].
These results could be explained by the fact that there was progressive oxidation during the period of storage. Caporaso et al., 2013 reported that polyphenols play an important role in protecting olive oil against oxidation especially if olive oil is stored in dark conditions.
Comparing between total phenol content of VOO samples with natural additives with those VOO used as a packing medium for concentrated Yoghurt balls (Table7), it was clear that the packing VOO had lower total phenol content. These results revealed the higher autoxidation which accelerated by the presence of humidity in concentrated Yoghurt balls [3].
Conclusion
The principal causes of deterioration of the virgin olive oil during storage are the oxidation reactions and their products as well as the loss of natural antioxidants. Acidity in all VOO samples with the different additives, whether, those used as a packing medium for traditional concentrated Yoghurt balls or the others without concentrated Yoghurt balls, were less than the acidity of the control sample. In addition, values of peroxide values exceed the limited value of Palestinian standard for all samples with the natural additives for VOO, but less than the control at the end of storage time. For Tomato Peel and Chili Pepper additives, the lower concentration of natural additives gives better results than the higher concentration. K232 and K270 for VOO values didn’t exceed the legislation limit accepted for VOO during 6 months of storage time for all samples of VOO with the natural additives. For VOO samples used for packing traditional concentrated Yoghurt, K232 and K270 values exceeded the official limit for Arum and tomato peel added samples. On the other hand, K232 for the chili pepper additives remained lower than the accepted limit for VOO. At the end of the storage period of six months, the total phenol content for samples used as a packing medium for the traditional concentrated Yoghurt was lower than the samples of olive oil with the natural antioxidants stored without concentrated Yoghurt balls. However, all natural additives used in this investigation showed a positive trend for improving the quality of olive oil used as a packing medium for traditional concentrated Yoghurt in different rates.
Acknowledgments
The authors wish to express their gratitude to the staff in Al-Jebrini Company for food Industries for their help in running storage simulation and samples analysis namely Mr Romel Qudemat.
References
- Aliakbarian, B, De Faveri, D, Converti, A, Perego, P., Biochemical Engineering Journal, 2008, 42, 34-40.
CrossRef - Bendini, A., Cerretani, L., Carrasco-Pancorbo, A., Gómez- Caravaca, A.M., Segura-Carretero, A., Fernández-Gutiérrez, A. and Lercker, G., Molecules, 2007, 12, 1679-1719.
CrossRef - Boskou, D., (2006): “Olive oil chemistry and technology”, (2nd ed), AOCS press, Champaign, Illinois.
CrossRef - Krichene, D., Allalout, A, Mancebo-Campos, V. Salvador, M.D.; Zarrouk M., Fregapane G. Food Chemistry, 2010, 121, 171–17.
CrossRef - Servili, M., Baldioli, M., Mariotti, F., Montedoro, G.F. Acta Horticulturae, 1999, 474, 609-613.
CrossRef - Okogeri, O. & Margari, M.T. Journal of Agricultural and Food Chemistry, 2002. 50, 1077-1080.
CrossRef - Brenes, M., Garcia, A., Garcia, P. & Garrido, A. Journal of agricultural and food chemistry, 2001. 49, 5609-5614.
CrossRef - Clinquants, L., Esti, M. & La Notte, E. Journal of the American Oil Chemists’ Society, 1997. 74, 1259–1264.
CrossRef - Tanaka, M., Kuei, C.W., Nagashima, Y., Taguchi, T. Nippon Suisan Gakkaishi, 1998, 54, 1409–1414.
CrossRef - Valdés, V., Mellinas, A.C., Ramos, M., Garrigós, M.C. and Jiménez, A. Front. Chem., 2014, 2, 1-6.
CrossRef - Rather, S. A., Masoodi, F. A.; Akhter, R., Rather, J. A. and Shiekh, K.A., 2016:” Advances in use of natural antioxidants as food additives for improving the oxidative stability of meat Products”. Department of Food Science and Technology, University of Kashmir, Srinagar-190006, India.
CrossRef - Caporaso,.N., Paduano, A, Sacchi,.R. European Journal of Lipid Science and Technology December 2013, 115 (12), 512-517.
CrossRef - Shtayeh, A., and Jamous, R.M., European Journal of Integrative Medicine, 2011. 3(3): 129-130.
CrossRef - George, B., Kaur, C., Khurdiya, D. S., & Kapoor, H. C. Food Chemistry, 2004, 84, 45–51.
CrossRef - Hasler, C. M., Food Technol. 1998, 52, 63–69.
CrossRef - Hyeon Sim, K., Young Sil, H., Int. J. Food Sci. Technol. 2008, 43, 1813–1823.
CrossRef - Liang Si. W. Y., Ma, K. Y., Chung, H. Y., Chen, Z.‐Y., J. Agric. Food Chem. 2012, 60, 6230–6234.
CrossRef - Perucka, I., Materska, M.G. Food Sci. Emerg. Tech., 2001, 2: 189–192.
CrossRef - Ahmad, S., Umar, H., Anjum, F. M., and Anjum, M. Buffalo Bulletin 2013 .32, 1316-1323.
- Ozgur, M.O., Akpinar-Bayizit, T. A. and Yilmaz-Ersan, L. ” African Journal of Agricultural Research 2011, 6(25), 5638-5644.
- Benakmoum, A., Abbeddou, S., Ammouche, A., Panagiotis Kefalas, Dimitrios Gerasopoulos: Food Chemistry, 2008, 110, 684–690.
CrossRef - Borel, P., Grolier, P., Armand, M., Partier, A., Lafont, H., Lairon, D. Journal of Lipid Research, 1996, 37, 250–261.
- Korel, F., Badatliolu, N., Balaban, M., Hiil, Y., J. Agric. Food Chem. 2009, 50, 3257–3261.
CrossRef - Montesano, D., Cossignani, L., D’Arco, G., Simonetti, M. S., & Damiani, P. JAOCS, 2006, 83, 933–941.
CrossRef - Taghvaei, M., Jafari, S.M. (2013). Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives, e: 6 July 2013 # Association of Food Scientists & Technologists (India) 2013.
- Gómez-Alonso, S. Mancebo-Campos, V.; Salvador, M. D.; Fregapane, G., Food Chem., 2007, 100, 36–42.
CrossRef - Caponio, F., Bilancia, B.T., Pasqualone, A., Sikorska, E. & Gomes, T., European Food Research and Technology, 2005, 221-226.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









