Investigation of Specific Interactions between the Constituent Molecules of Binary Liquid Mixtures of Methyl Benzoate, Ethyl Benzoate and Benzyl Benzoate with 2- Pentanol at Different Temperatures
Kailas Kapadnis1 , Kailaspati Jadhav1
, Kailaspati Jadhav1 and Pankaj Pawar2
and Pankaj Pawar2
1Research Centre in Chemistry, M.G. Vidyamandir’s L.V.H. College Nashik- 422003, Maharashtra, India. Affiliated to S. P. Pune University, Pune, India.
2Department of Chemistry, M. S.G. College, Malegaon Camp, India (M.S.), Pin 423105
Corresponding Author E-mail: kkjadhav67@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360320
Article Received on : 10 May 2020
Article Accepted on : 11 Jun 2020
Article Published : 08 Jun 2020
The current work focuses on the understanding of viscosity, density and ultrasonic velocity and their deviation of binary liquid mixtures of Methyl benzoate, Ethyl benzoate and Benzyl benzoate with 2 Pentanol at temperature 298.15K and 308.15K.The composition of liquid mixtures is taken in terms of mole fraction from 0.1 to 1.0.From these data, excess molar volume, deviation in viscosity and isentropic compressibility have been calculated. These calculated quantities have been utilized in Redlich-Kister equation to get the coefficients and standard errors. These parameters for the liquid mixtures have been adopted in the study of the molecular interactions and the effects of methyl, ethyl and benzyl group of benzoates present on benzene ring.
KEYWORDS:Deviation In Viscosity; Excess Molar Volume; Isentropic Compressibility; Molecular Interactions; Mole Fraction
Download this article as:| Copy the following to cite this article: Kapadnis K, Jadhav K, Pawar P. Investigation of Specific Interactions between the Constituent Molecules of Binary Liquid Mixtures of Methyl Benzoate, Ethyl Benzoate and Benzyl Benzoate with 2- Pentanol at Different Temperatures. Orient J Chem 2020;36(3). |
| Copy the following to cite this URL: Kapadnis K, Jadhav K, Pawar P. Investigation of Specific Interactions between the Constituent Molecules of Binary Liquid Mixtures of Methyl Benzoate, Ethyl Benzoate and Benzyl Benzoate with 2- Pentanol at Different Temperatures. Orient J Chem 2020;36(3). Available from: https://bit.ly/2AKkjO2 |
Introduction
Molecular interactions in binary liquid mixtures have been playing a crucial role in laboratory research since long back.1-2 2-Pentanol is having many uses such as food additive, food additive flavour, antifreeze agent, cleaning agent, ion exchange agent, fuel, fuel additives, in industrial gas manufacturing and petroleum refineries. Methyl benzoate, ethyl benzoate and benzyl benzoate being polar solvents are utilized in many designing applications. Methyl and ethyl benzoate and benzyl benzoate have been broadly used in the flavouring, perfumery, artificial essences and cosmetics. Numerous works have been performed on the binary mixtures of above said esters recently17-20 but no studies on properties such as density, viscosity and ultrasonic velocity for binary mixture of these esters with 2- pentanol have been accounted so far in literature survey. Calculations of density, viscosity and ultrasonic velocity could find broad applications in making characterization the thermodynamic and physico- chemical aspects of binary liquid mixtures as the constituent components have wide applications in industry and other fields as disclosed earlier. The main intention of this study is to elaborate molecular interactions by using excess functions such as excess volume, deviation in viscosity and isentropic compressibility factor in the studied binary liquid mixtures.
Materials and Methods
The solvent used was 2-Pentanol and imported from Sigma Germany having purity 99%.The solutes are Methyl benzoate, Ethyl benzoate, and Benzyl benzoate of Acros having purity 99% were utilized after first distillation. Experimental values of density, viscosity and ultrasonic velocities of pure liquids are compared at 298.15K, 303.15K, and these values are demonstrating acceptable concurrence with literature values published.3-14 Specially designed stoppered bottles were used to prepare mixtures in terms of mole fractions. All the mixtures were utilized on same day for the estimations of above said parameters. Electronic balance of Adair Dutt with an accuracy of 0.0001 mg. was used to prepare the binary mixtures. Digital densitometer model number DMA 35-84138 manufactured by Anton Par with an accuracy of 0.001 gm/cm3, reproducibility of 0.0007 gm/cm3 having capacity 2 ml, was used to measure the densities of pure liquids and their binary mixtures. Digital viscometer model number LVDVII + Pro manufactured by Brookfield Engineering Laboratories, Middleboro INC [USA], calibrated with triply distilled water with an accuracy ± 1% of full scale of range and viscosity repeatability ± 2% , was used to measure the viscosities of pure liquid and their binary liquid mixtures. Variable path single crystal interferometer from Mittal Enterprises F-05(SI No.1415071) model, New Delhi having frequency 2 MHz (with precision of ± 0.8 meter per second), was used to measure ultrasonic velocities of pure liquids and its binaries. Calibration of interferometer was done by using triply distilled water, methanol and benzene.
Table 1: Comparison of experimental and literature values of density, viscosity and ultrasonic velocity of 2-Pentanol, Methyl benzoate, Ethyl benzoate and Benzyl benzoate at 298.15K and 308.15K
|
Sr.No |
Chemical |
Temp/K |
Density(gm.cm-3) |
Viscosity(mPas) |
Ultrasonic velocity(ms-1) |
|||
|
Expt |
lit |
Expt |
lit |
Expt |
lit |
|||
|
1. |
2-Pentanol |
298.15 |
0.8050 |
0.8054a,b,c |
3.273 |
3.478a,b,,c |
1232 |
1232j |
|
308.15 |
0.7984 |
0.7987c |
2.494 |
2.370i |
1197 |
– |
||
|
2. |
Methyl benzoate |
298.15 |
1.0832 |
1.0836d |
1.819 |
1.825d |
1406 |
1406d |
|
308.05 |
1.0736 |
1.0739e |
1.503 |
1.510d |
1370 |
1372e |
||
|
3. |
Ethyl benzoate |
298.15 |
1.0419 |
1.0413f |
1.932 |
1.936e |
1374 |
1378l |
|
308.15 |
1.0329 |
1.0325e |
1.594 |
1.591e |
1338 |
1338k |
||
|
4. |
Benzyl benzoate |
298.15 |
1.1226 |
– |
8.287 |
8.292g |
1530 |
– |
|
308.15 |
1.1139 |
1.1131h |
5.226 |
5.229h |
1482 |
1486h |
||
References:-a= 3, b= 4,c= 5,d= 6,e= 7,f= 8,g= 9,h= 10, i= 11, j=12,k= 13,l=14
Result and Discussion
The values of density, viscosity and ultrasonic velocity as a function of mole fractions obtained from an experiment at temperature 298.15 and 308.15K are clearly tabulated. The values of density are exercised to compute the excess molar volumes VE by employing the equation,
VE/ (cm3.mole-1) = (x1M1+ x2M2)/ ρ12 – (x1M1/ρ1) – (x2M2/ρ2) … (1)
Where ρ12 is the density of the mixture, x1, M1, ρ1 andx2, M2, ρ2 are the mole fractions, molecular weights and densities of pure components 1 and 2 respectively.
The deviations in viscosities ∆η were estimated by employing the relation,
∆η = η12 – x1η1 – x2η2 …… (2)
Whereη12 is the viscosity of the mixture, x1,x2 and η1, η2 are the mole fractions and viscosities of the pure components 1 and 2 respectively.
The excess isentropic compressibilities (Ks) were computated by employing the relation,
Ks = 1/u2p …… (3)
Where u is the ultrasonic velocity p is the density.
And the deviation in isentropic compressibilitis (∆Ks) were estimated by using the relation,
∆Ks = Ks (12) –x1 Ks1–x2Ks2…… (4)
Where Ks (12) is the compressibilities of the mixture, x1,x2 and, Ks1, Ks2 are the mole fractions and isentropic compressebilities of the pure components 1 and 2 respectively.
The excess molar volumes, deviation in viscosities and isentropic compressibilitis were put into Redlich Kister equation of following type,

Where Y is either, VE, ∆η, or ∆Ks and n is the degree of polynomial. Coefficients ai were sought by applying equation 5 to experimental results using a least-squares regression method. In each case, the numbers of coefficients are determined from the examination of variation in standard deviation (σ) and it was estimated by adopting the equation,
σ(Y) = [∑ (Yexpt– Ycal)2]1/2 / N-n …… (6)
Where N is the number of data points and n is the number of coefficients. The computated values of the coefficients (ai) along with the standard deviations (σ) are displayed in the table 6.
Hind et. al.offered an equation for the viscosity of binary liquid mixtures as,
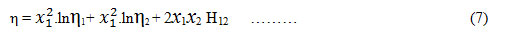
where x1, x2, η1, η2 are the mole fractions and viscosities of solvent and solute respectively and H12 is the interaction parameter.
Katti and Chaudhari suggested following equation;
log ( ηmVm) = x1log(η1V1) + x2log(η2V2) + x1x2[Wvis/ (RT)] …… (8)
where ηm, and Vm are the viscosity and volume of the mixture, Wvis is defined as interaction energy for the activation of flow.
The Jouyban and Acree proposed a model for correlating the density and viscosity of liquid mixtures at various temperatures. The equation is,
lnymT = f1 lny1, T + f2 lny2, T + f1f2 ∑ [ Aj (f1-f2)j /T ] ………… (9)
Where ymT , y1, T and y2,T is density or viscosity of the mixture and solvent 1 and 2 respectively at given temperature T and f1, f2 are the mole fractions and Aj are model constants.
Jouyban – Acree model is applied to the density, viscosity and speed of sound of data and the correlating ability of this model was tested by calculating the average percent deviation (APD) between the experimental and calculated density, viscosity and speed of sound as
APD = (100/N) ∑ [(y expt – y calc) / y expt] ………… (10)
Where N is the number of data points in each set, y represents density or viscosity. The optimum number of constants Aj and in each case they were determined from the examination of APD values.
Density, viscosity, ultrasonic velocity, deviation in viscosity, excess volume and compressibility factor for binary liquid mixtures containing 2- pentanol with methyl benzoate, ethyl benzoate and benzyl benzoate at 298.15K and 308.15K have been computed over entire range of composition of mole fractions and shown in table 2,3 and 4. Parameters of Jouyban-Acree model and average percentage deviation for density, viscosity and ultrasonic velocity are also disclosed in table 5 while interaction parameters for the binary system in table 6.
Table 2: Experimental values of density (r), viscosity (η), excess molar volume (VE) deviations in viscosity (∆η), isentropic compressibility (∆Ks) for 2- Pentanol + Methyl benzoate
|
Temp/K |
x1 |
ρ x10-3 Kg.m-3 |
η mPa.s |
U m/s |
VEx106 m3.mol-1 |
∆η mPa.s |
∆Ks Tpa-1 |
|
298.15K |
0.0000 |
1.0832 |
1.819 |
1406 |
0.000 |
0.000 |
0.000 |
|
0.0992 |
1.0562 |
1.655 |
1364 |
0.311 |
-0.308 |
7.200 |
|
|
0.2009 |
1.0290 |
1.531 |
1330 |
0.502 |
-0.580 |
12.500 |
|
|
0.2994 |
1.0025 |
1.404 |
1302 |
0.630 |
-0.850 |
15.900 |
|
|
0.4002 |
0.9752 |
1.281 |
1279 |
0.701 |
-1.120 |
18.500 |
|
|
0.5091 |
0.9454 |
1.169 |
1261 |
0.715 |
-1.390 |
20.300 |
|
|
0.6007 |
0.9199 |
1.178 |
1249 |
0.693 |
-1.514 |
19.200 |
|
|
0.6984 |
0.8924 |
1.304 |
1240 |
0.615 |
-1.530 |
16.900 |
|
|
0.8003 |
0.8631 |
1.643 |
1234 |
0.502 |
-1.340 |
12.100 |
|
|
0.8998 |
0.8342 |
2.222 |
1232 |
0.310 |
-0.905 |
7.200 |
|
|
1.0000 |
0.8050 |
3.273 |
1232 |
0.0000 |
0.000 |
0.000 |
|
|
308.15K |
0.0000 |
1.0736 |
1.503 |
1370 |
0.000 |
0.000 |
0.000 |
|
0.0992 |
1.0460 |
1.310 |
1326 |
0.422 |
-0.291 |
10.000 |
|
|
0.2009 |
1.0191 |
1.202 |
1289 |
0.616 |
-0.500 |
18.900 |
|
|
0.2994 |
0.9931 |
1.075 |
1260 |
0.721 |
-0.725 |
25.100 |
|
|
0.4002 |
0.9661 |
1.005 |
1237 |
0.794 |
-0.895 |
28.900 |
|
|
0.5091 |
0.9365 |
0.949 |
1218 |
0.825 |
-1.059 |
31.000 |
|
|
0.6007 |
0.9114 |
0.981 |
1207 |
0.789 |
-1.117 |
29.400 |
|
|
0.6984 |
0.8841 |
1.073 |
1200 |
0.724 |
-1.122 |
25.000 |
|
|
0.8003 |
0.8554 |
1.301 |
1196 |
0.572 |
-0.995 |
18.600 |
|
|
0.8998 |
0.8269 |
1.737 |
1196 |
0.367 |
-0.658 |
9.900 |
|
|
1.0000 |
0.7984 |
2.494 |
1197 |
0.000 |
0.000 |
0.0000 |
Table 3: Experimental values of density (r), viscosity (η), excess molar volume (VE), deviations in viscosity (∆η) and isentropic compressibility (∆Ks) for 2- Pentanol + Ethyl benzoate
|
Temp/K |
x1 |
ρx10-3 Kg.m-3 |
η mPa.s |
U m/s |
VEx106 m3.mol-1 |
∆η mPa.s |
∆Ks Tpa-1 |
|
298.15K |
0.0000 |
1.0419 |
1.932 |
1374 |
0.000 |
0.000 |
0.000 |
|
0.0992 |
1.0214 |
1.738 |
1341 |
0.258 |
-0.329 |
5.300 |
|
|
0.2009 |
1.0008 |
1.647 |
1312 |
0.437 |
-0.554 |
9.500 |
|
|
0.2994 |
0.9794 |
1.531 |
1288 |
0.563 |
-0.805 |
13.400 |
|
|
0.4002 |
0.9575 |
1.439 |
1269 |
0.632 |
-1.030 |
15.600 |
|
|
0.5091 |
0.9352 |
1.345 |
1252 |
0.664 |
-1.255 |
16.400 |
|
|
0.6007 |
0.9109 |
1.376 |
1243 |
0.650 |
-1.361 |
15.800 |
|
|
0.6984 |
0.8861 |
1.487 |
1236 |
0.584 |
-1.384 |
13.800 |
|
|
0.8003 |
0.8605 |
1.779 |
1232 |
0.468 |
-1.224 |
9.300 |
|
|
0.8998 |
0.8331 |
2.357 |
1231 |
0.312 |
-0.781 |
5.100 |
|
|
1.0000 |
0.8050 |
3.273 |
1232 |
0.0000 |
0.000 |
0.000 |
|
|
308.15K |
0.0000 |
1.0329 |
1.594 |
1338 |
0.000 |
0.000 |
0.000 |
|
0.0992 |
1.0121 |
1.423 |
1301 |
0.332 |
-0.262 |
9.000 |
|
|
0.2009 |
0.9916 |
1.334 |
1271 |
0.528 |
-0.440 |
16.600 |
|
|
0.2994 |
0.9705 |
1.255 |
1247 |
0.644 |
-0.610 |
22.100 |
|
|
0.4002 |
0.9487 |
1.174 |
1227 |
0.731 |
-0.780 |
26.100 |
|
|
0.5091 |
0.9267 |
1.142 |
1210 |
0.752 |
-0.900 |
30.200 |
|
|
0.6007 |
0.9026 |
1.173 |
1202 |
0.745 |
-0.961 |
26.400 |
|
|
0.6984 |
0.8781 |
1.255 |
1196 |
0.671 |
-0.969 |
21.900 |
|
|
0.8003 |
0.8527 |
1.466 |
1194 |
0.562 |
-0.847 |
16.100 |
|
|
0.8998 |
0.8258 |
1.821 |
1194 |
0.373 |
-0.582 |
9.000 |
|
|
1.0000 |
0.7984 |
2.494 |
1197 |
0.000 |
0.000 |
0.000 |
Table 4: Experimental values of density (r), viscosity (η), excess molar volume (VE), deviations in viscosity (∆η) and isentropic compressibility (∆Ks) for 2- Pentanol + Benzyl benzoate
|
Temp/K |
x1 |
ρx10-3 Kg.m-3 |
η mPa.s |
U m/s |
VEx106 m3.mol-1 |
∆η mPa.s |
∆Ks Tpa-1 |
|
298.15K |
0.0000 |
0.0000 |
8.287 |
1530 |
0.000 |
0.000 |
0.000 |
|
0.0992 |
0.0986 |
7.434 |
1448 |
0.342 |
-0.359 |
9.400 |
|
|
0.2009 |
0.1985 |
6.661 |
1384 |
0.523 |
-0.631 |
16.700 |
|
|
0.2994 |
0.3009 |
5.865 |
1333 |
0.662 |
-0.913 |
20.900 |
|
|
0.4002 |
0.3992 |
5.115 |
1296 |
0.725 |
-1.170 |
23.900 |
|
|
0.5091 |
0.5000 |
4.365 |
1265 |
0.763 |
-1.415 |
24.800 |
|
|
0.6007 |
0.5993 |
3.713 |
1244 |
0.756 |
-1.569 |
23.300 |
|
|
0.6984 |
0.7004 |
3.185 |
1229 |
0.673 |
-1.590 |
20.100 |
|
|
0.8003 |
0.7994 |
2.895 |
1222 |
0.547 |
-1.384 |
16.800 |
|
|
0.8998 |
0.9006 |
2.807 |
1222 |
0.335 |
-0.964 |
9.500 |
|
|
1.0000 |
1.0000 |
3.273 |
1232 |
0.000 |
0.000 |
0.000 |
|
|
308.15K |
0.0000 |
1.1139 |
5.226 |
1482 |
0.000 |
0.000 |
0.000 |
|
0.0992 |
1.0926 |
4.686 |
1399 |
0.417 |
-0.271 |
13.200 |
|
|
0.2009 |
1.0705 |
4.182 |
1335 |
0.621 |
-0.502 |
23.200 |
|
|
0.2994 |
1.0462 |
3.673 |
1285 |
0.751 |
-0.731 |
30.000 |
|
|
0.4002 |
1.0209 |
3.181 |
1248 |
0.823 |
-0.954 |
34.000 |
|
|
0.5091 |
0.9925 |
2.699 |
1220 |
0.857 |
-1.161 |
35.800 |
|
|
0.6007 |
0.9618 |
2.292 |
1200 |
0.833 |
-1.297 |
34.200 |
|
|
0.6984 |
0.9271 |
1.985 |
1188 |
0.768 |
-1.328 |
30.100 |
|
|
0.8003 |
0.8894 |
1.854 |
1182 |
0.618 |
-1.188 |
23.600 |
|
|
0.8998 |
0.8460 |
2.000 |
1185 |
0.403 |
-0.766 |
13.600 |
|
|
1.0000 |
0.7984 |
2.494 |
1197 |
0.000 |
0.000 |
0.000 |
Table: 5: Parameters of Jouyban-Acree model and average percentage deviation for density, viscosity and ultrasonic velocity for the binary systems
|
System- 2 Pentanol + |
A0 |
A1 |
A2 |
A3 |
A4 |
APD |
|
Density |
||||||
|
Methyl benzoate |
17.8036 |
3.5778 |
-3.9143 |
– |
– |
0.0112 |
|
Ethyl benzoate |
24.5139 |
4.8573 |
-1.9546 |
– |
– |
0.0063 |
|
Benzyl benzoate |
62.7306 |
21.9791 |
6.3680 |
2.8598 |
-1.7017 |
0.0086 |
|
Viscosity |
||||||
|
Methyl benzoate |
-856.4083 |
-481.0626 |
133.2435 |
295.5697 |
-166.3345 |
0.7869 |
|
Ethyl benzoate |
-695.6763 |
-395.7238 |
41.3756 |
244.2325 |
-115.0130 |
0.7600 |
|
Benzyl benzoate |
-294.4104 |
-531.1567 |
-399.2081 |
2.7034 |
85.9469 |
1.3772 |
|
Ultrasonic Velocity |
||||||
|
Methyl benzoate |
-57.8511 |
8.6331 |
-0.6037 |
1.7135 |
3.4310 |
0.1118 |
|
Ethyl benzoate |
-51.4108 |
4.9804 |
6.9962 |
0.7109 |
-5.4495 |
0.1049 |
|
Benzyl benzoate |
-106.5277 |
11.1042 |
-13.4274 |
2.1571 |
– |
0.1324 |
Table 6: Interaction parameters for the binary system
|
System- 2-Pentanol + |
Temp/K |
d |
σ |
W visc/RT (kj.mol-1) |
σ |
H12 mPa.s |
σ |
|
Methyl benzoate |
298.15 |
-2.809 |
0.13 |
-2.933 |
0.14 |
-0.344 |
0.220 |
|
308.15 |
-2.788 |
0.06 |
-2.739 |
0.06 |
-0.232 |
0.110 |
|
|
Ethyl benzoate |
298.15 |
-2.427 |
0.10 |
-2.503 |
0.11 |
-0.037 |
0.170 |
|
308.15 |
-2.229 |
0.05 |
-2.156 |
0.05 |
0.122 |
0.090 |
|
|
Benzyl benzoate |
298.15 |
-0.918 |
0.13 |
-0.914 |
0.14 |
2.747 |
0.100 |
|
308.15 |
-1.395 |
0.11 |
-1.213 |
0.10 |
1.370 |
0.080 |
Where d, W visc, H12 are the interaction parameters and σ is the standard deviations .
The excess volume variation (disparity) with mole fraction x1 of 2-Pentanol with methyl benzoate, ethyl benzoate and benzyl benzoate at 398.15K is clearly shown in fig (1). Literature15-18 gives excess volume variation of methyl benzoate and ethyl benzoate with alcohols at 298.15K but no data have been reported so far for methyl benzoate and ethyl benzoate with 2-pentanol. It has been observed from the data and graphical representation that excess volume and deviation in compressibility factor show positive deviation with maxima at mole fraction 0.5 for 2-Pentanol with methyl benzoate, ethyl benzoate and benzyl benzoate system over entire range of composition of mole fraction in figure 1 and 3 whereas deviation in viscosity for all systems are showing negative deviation with minima at mole fraction 0.7 depicted in figure 2 and it has been observed that ∆η are negative for all systems showing the weak interactions present in the binary mixtures under consideration. ∆η values slightly increase with increase in temperature,19 and are negative for all systems showing the weak interactions present in the binary mixtures and the credit of such behaviour goes to breaking of dipole-dipole interaction as well as interactions formation between the polar benzoate group and electron donating methyl, ethyl and benzyl group. The trend of excess volume is, 2-pentanol + benzyl benzoate > 2-pentanol + methyl benzoate > 2 -pentanol + ethyl benzoate. It has been also shown that excess volume increases with increase in temperature for all three binary systems.
 |
Figure 1: Plot of excess molar volume against mole fraction of 2- pentanol |
 |
Figure 2: Plot of deviation in viscosity against mole fraction of 2- pentanol (x1) |
 |
Figure 3: Plot of deviation in compressibility factor against mole fraction |
Conclusion
Calculations of excess volume, deviation in viscosity and deviation in compressibility factor derived from density, viscosity and ultrasonic velocity are clearly indicating the weak interactions are present in binary liquid mixtures at specific composition. The knowledge of these interactions could be used in many industrial applications.
Acknowledgement
The completion of this work could not have been possible without the assistance and guidance of the Principal and Head of the department LVH College of Arts, Commerce and Science College, Panchavati, Nashik-3. Authors also wish to express our sincere gratitude to them for extending their cooperation and facilities provided to embark this work.
Conflict of Interest
The authors declare no conflict of interest.
References
- Aralaguppi, M.I.; Aminabhavi, T.M.; Balgundi, R.H. Fluid Phase Equilib, 1992, 71, 99.
- Joshi, S.S.; Aminabhavi, T.M. Fluid Phase Equilib, 1990, 60, 319.
- Iloukhani, H; Almasi, M. Thermochemical Acta, 2009, 495, 139-148.
- Almasi, M.; Iloukhani, H. Chem. Eng. Data, 2010, 55 1416-1420.
- D’Aprano.; A.Donato.; Agriento, V. Solution Chem. 1981,9, 673-680.
- Aminabhavi, T.M.; Raikar, S.R.; Balundgi, R.H. Chem. Eng. Data, 1993,38, 441.
- Aminabhavi, T.M.; Phayde, H.T.S.; Khinnavar, R.S.; Gopalakrishna, B.J.; Chem. Eng. Data, 1994,39, 251.
- Lien Pei-Jung,; Lin Ho-Mu,; Lee Ming-Jer,; Venkatesu, P. Fluid Phase Equilib, 2003,206, 105.
- Riddick J.A,; Bunger W.B,; Sakano, T.K. Organic Solvents II, 430.
- Sankar, S.J,; Geeta , L.M,; Naidu, P.S,; Prasad, K.R. Pure Appl. Ultrason, 29,2007,82.
- Almasi, M,; Chem.Res.2019, 7 (2), 365-374.
- Gonzalez, B,; Dominguez, A,; Tojo, J. Chem.Eng.Data, 2006 51,1076-1087.
- Aminabhavi, T.M,; Phayde, H.T.S,;.Khinnavar, R.S,; Gopalakrishna, B. Chem. Eng. Data, 39, 1994, 251.
- Nagarjun, B,; Sharma, A.V,;.Ramarao, G.V,; Rambhau, C. Thermodynamics, 2013, 1-9
- Grunberg, L,; Nissan, A. Nature, 1949, 164 799-800
- Sastry,S.S,; Babu Shaik,; Vishwam, T,; Sie Tiong Ha. Physics and Chemistry of Liquids, 2014, 52(2), 272-286.
- Sastry, S.S,; Babu Shaik,;Vishwam,T,; Sie Tiong Ha. Physica B: Condensed Matter, 2013,420, 40-48.
- Sastry,S.S,; Babu Shaik,; Vishwam,T,; Sie Tiong Ha. Thermal Analysis and Calorimetry, 2014, 116 (2),923-935.
- Sastry, S.S,; Shaik.Babu,;Vishwam, T. Physics and Chemistry of Liquids, 2014, 52 (2), 272-286.
- Paez,S,; Contreras, M. Chem. Eng. Data, 1989, 34, 455-459.

This work is licensed under a Creative Commons Attribution 4.0 International License.









