Removal of Chromium Ions from Tannery Wastewater using Cactus Powder
Waleed H. Rimawi* , Safa Shaheen and Hatim Salim
, Safa Shaheen and Hatim Salim
Department of Applied Chemistry, Palestine Polytechnic University, Hebron P.O. Box 198, Palestinian Territories.
Corresponding Author E-mail: whrimawi@ppu.edu
DOI : http://dx.doi.org/10.13005/ojc/360118
Article Received on : 01 Nov 19
Article Accepted on :
Article Published : 13 Feb 2020
Leather industry consumes large amounts of chemicals, particularly toxic chromium sulfate. 30-40% of chromium used in this industry is released as a solid or liquid waste, which eventually ends up in soil and ground water. This work aims at determining the total chromium in samples of tannery wastewater and removing it using Cactus as adsorbent. Detection of chromium (VI) and total chromium was performed spectrophotometricaly using 1.5-diphenylcarbazide reagent in addition to diazo-coupling reaction (between sulphanilic acid and N, N-dimethylaniline). The efficiency of removing Chromium ions from contaminated tannery wastewater by adsorption on cactus powder was investigated by batch experiments and the effects of various parameters (pH, adsorbent dosage and treatment time) on the percentage removal of chromium were investigated. The percentage removal obtained was 98.8% for chromium (VI) and 97.0% for chromium (III) at optimum conditions: pH = 8, adsorbent dosage equal 6.0 g/l and contact time of 60 min. The results of this work demonstrated for first time that removing chromium ions, especially carcinogenic chromium (VI), using cactus powder is more efficient than commonly used calcium carbonate.
KEYWORDS:Adsorption; Cactus; Tannery Wastewater; Total Chromium.
Download this article as:| Copy the following to cite this article: Rimawi W. H, Shaheen S, Salim H. Removal of Chromium Ions from Tannery Wastewater using Cactus Powder. Orient J Chem 2020;36(1). |
| Copy the following to cite this URL: Rimawi W. H, Shaheen S, Salim H. Removal of Chromium Ions from Tannery Wastewater using Cactus Powder. Orient J Chem 2020;36(1). Available from: https://bit.ly/2UNFHdl |
Introduction
As it is known, the leather industry consumes large quantities of water. Large amounts of different types of chemical compounds utilized in leather manufacturing processes are not completely fixed or exhausted by the skins, resulting in high chemical content in the tannery effluent. These chemicals are discharged as a waste in tannery wastewater, leading to several environmental threats in the absence of proper treatment and disposal. The tannery wastewater is highly contaminated by various chemical pollutants including organic and inorganic compounds and salts, that results in their high toxicity and chemical oxygen demand (COD) [1, 2]. Chromium is one of major chemical constituents of the tannery wastewater with concentrations varying from 1500 to 3000 mg/l [3], where this concentration in tannery wastewater according to Germany standards does not exceed the value 0.05 mg/L for Cr (VI) and 1 mg/L for total chromium [4]. Chromium in nature can be found in different chemical forms, however Cr (VI) in the forms of CrO42−, Cr2O72− and CrO3 is the most toxic among others, because of its strong oxidizing power, water solubility, and ability to pass though biological membranes and it is known as inhalation irritant and associated with respiratory cancer [5-10].
Researchers have been intensively studying the treatment of tannery wastewater to remove chromium ions using several techniques, such as chemical coagulation [3], Sedimentation techniques, [11], coagulation-flocculation methods [12], treatment with lime and activated carbon [13], adsorption onto bentonite [14], amine-modified polyacrylamide [15], etc. However adsorption techniques are the most widely used for chromium removal from industrial wastewaters [4] due to their significant advantages like low cost, abundance, simplicity of use and efficiency, compared with other methods [16, 17]. Large number of adsorbents are used for the treatment of tannery wastewater, some are natural and others are synthetic [4, 18].
Cactus (Fig 1) is well studied for its properties and composition [19-24] and widely used as biosorbent in removing different contaminants and metal ions from wastewater samples [18, 19, 25-28]. In addition to its advantages like low cost, ease of use, abundance and low sludge mass it is absolutely an eco-friendly biosorbent and safe for human health. In Palestine Cactus is historically one of the most commonly grown plants. Therefore, this work aims at studying the removal efficiency of Chromium (Cr (VI), Cr (III)) from local tanneries wastewater using Opuntia ficus-indica Cactus and comparing it with the commonly used Calcium carbonate.
 |
Figure 1: Cactus Opuntia ficus-indica (p.cuctaceae) plant. |
Materials and Methods
Materials
The reagents and calibration solutions were prepared from the following chemicals: acetone (Sigma Aldrich, ≥99% purity), glacial acetic acid (Sigma Aldrich, ≥99% purity), 1,5-diphenylcarbazide (alpha Acer, ≥99% purity), H2SO4 (Sigma Aldrich, 95%-97% purity), K2CrO4 (Sigma Aldrich, ≥99% purity) and K2Cr2O7 (Sigma Aldrich, ≥99% purity).
For preparation of N, N-dimethylaniline (0.1%) 0.1 ml of pure reagent was mixed with 4 mL of 3N HCI and diluted to 100 ml of water. Saturated bromine water (3% w/v), (5 %) solution of sulphosalicylic acid, 4 M KOH, H2SO4 (2.5M), 3 M HCI, 4 M NaOH, acetate buffer (PH=4), hydroxylamine hydrochloride (0.1%) and sulphanilic acid (0.05%) were also prepared.
Tannery wastewater samples were obtained from Al-Za’tari tanneries in industrial area in Hebron/Palestine. Triplicate samples were taken from the tanning process effluent at different height levels of collecting container into clean sterile 500-ml glass bottles and stored at room temperature till analysis. As reported in an earlier work [29], performed by our colleagues from Palestine Polytechnic University, samples of tannery wastewater from the same local leather factory were characterized as follows (COD-7.39 mg/l, TDS-60.3mg/l, TSS-17.3 mg/l).
Cactus Opuntia ficus-indica (p.cuctaceae) pads were collected from Al-daheriah-Hebron (31°24′27″N 34°58′20″E) in Palestine. Pads of Cactus were cut into thin slides, dried for 4 weeks under sun and then at 60 Cº in oven till constant mass. The obtained mass (127g) was then grinded for 4 min and the obtained powder was kept at –20°C.
Methods
Determination of Cr (VI) Concentration in Wastewater (Before Treatment with Cactus Powder)
Determination Cr (VI) was performed using the procedure described in [30]. 5 ml of wastewater was transferred to volumetric flask, sulfuric acid was to adjust pH at the value of 1.03, to the obtained solution 2 ml of Ligand (1.5-diphenyl carbazide) was transferred then distilled water was added to achieve the volume of 50 ml. Absorbance was read at 540 nm for each solution. Then the concentration of Cr (VI) was determined using the calibration curve constructed by measuring the absorbance values for solutions with concentrations of 0.1, 0.3, 0.5, 0.7, 0.9 and 1 ppm Cr (VI) after treatment according to the procedure above.
Determination of the Effect of pH on Cr (VI) Removal Using Cactus Powder or Calcium Carbonate as Adsorbents
Eight samples with the volume of each 33 ml of wastewater were transferred to beakers, then pH of solutions was adjusted to 2, 3, 5, 6, 7, 8, 9 and 10, after that the adsorbent (0.198 g) was added to each. The solutions were stirred for 1 hour and stand overnight at room temperature, and then filtered. The concentration of Cr (VI) ions was measured in the supernatant using the procedure described above. The same procedure was applied for studying the Cr (VI) removal using Calcium Carbonate as sorbent.
Determination of the Effect of Adsorbent Dosage on Cr (VI) Removal (Using Cactus Powder)
Nine different samples (0.066, 0.132, 0.198, 0.264, 0.330, 0.396, 0.436, 0.528 and 0.594 g) of adsorbent were transferred to beakers containing 33 ml wastewater each. Solutions were stirred for 1hour at room temperature and pH of 8 and let stand overnight. Then the solutions were filtered and the concentration of Cr (VI) was measured using the procedure described above.
Determination of the Effect of Contact Time on Cr (VI) Removal (Using Cactus Powder)
The adsorbent (0.198 g) was transferred to 33 ml of wastewater. The solution was allowed to stir at pH equal 8 and room temperature. Samples of supernatant were separated after different periods (30, 60, 90, 120, and 150 minutes). Samples were filtered and the concentration of Cr (VI) ions was measured using the procedure described above.
Determination of Total Chromium Concentration in Wastewater
Theconcentration of total chromium in wastewater was determined basing on the procedure presented in [8]. Accordingly, 500 µg of wastewater was taken and transferred to 50ml Erlenmeyer flask then 0.5 mL of 4M KOH and 1.5 ml of saturated bromine water were added and left for 5 min. Then 2.5 M H2SO4 (0.5 ml) and 5% sulphosalicylic acid (0.5 ml) were added. pH was adjusted to 4 using 3.5 ml of acetate buffer and hydroxylamine hydrochloride (0.1%) was added. After 5 min sulphanilic acid (0.05%) was added, the solution was left for 3 min, after what 0.1% N, N-dimethylaniline (5 ml) and 4 M NaOH (4 ml) were added. Then to the obtained solution 3N HCl was added and the volume was made up to 50 ml using distilled water. The absorbance was read at 507 nm for each solution using UV-Visible Spectrophotometer. For determination the total chromium concentration, initially standard Cr (III) solution with the concentration of (1 mg/l) was prepared by adding 0.28230 g of K2Cr2O7 to 50 ml of distilled water, then saturated solution of Na2S (1 ml) and 1 ml of 2.5 M H2SO4 were added, then the mixture was boiled for 5 min and diluted to 100 ml [31]. From the obtained solution a series of 10, 20, 30, 40, 50, 60, 70 and 80 µg/l Cr (III) solutions were prepared and treated as described in the procedure above. The Absorbance was read at 507 nm for each solution using UV-Visible Spectrophotometer and a calibration curve was constructed.
Removal of Total Chromium from Tannery Wastewater Samples Using Cactus powder
The pH of 33 ml of wastewater in a beaker was adjusted at 8 then 0.198 g of cactus powder was added. The solution was stirred for an hour and then allowed to stand overnight. 500 µg/l of supernatant was taken from the sample and transferred to 50-ml Erlenmeyer flask, then the total chromium concentration was measured using to the procedure described above.
Results and Discussions
Determination of Cr (VI) Concentrations in Tannery Wastewater before Treatment
The first step of this work was determining the concentration of Cr (VI) in tannery wastewater. This was performed spectrophotometricaly using the method developed in literature [30]. According to this method, 1.5-diphenylcarbazide is oxidized to 1.5-diphenylcarbazone by the action of Cr (VI) in acidic media producing a complex with chromium having red-violet color. The produced complex absorbs visible light with the wavelength of 540 nm. For quantitative determination of Cr (VI) concentration a calibration curve was constructed. For this purpose, absorbance was measured for Cr (VI) solutions of different concentrations after adding the ligand (1.5-diphenyl carbazide) to each solution. The obtained values of absorbance were plotted against concentration of Cr (VI) (ppm). The curve obtained was linear with correlation coefficient R2 equal 0.9959, which indicated a positive linear relationship between the parameters (Figure 2).
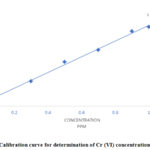 |
Figure 2: Calibration curve for determination of Cr (VI) concentration |
As a result of the work, the concentration of Cr (VI) in tannery wastewater obtained from local Palestinian Leather factory was determined to be 8.1 ppm. This value exceeds the level declared by International environmental standards according to which the chromium concentration in wastewater should not exceed 5 ppm for Cr (III) and 0.05 ppm for Cr (VI) [31]. This result confirms the need for treatment of tannery wastewater using technically feasible, cheap and environmentally safe method for removing toxic chromium ions.
Removal of Cr (VI) from Tannery Wastewater Using Cactus Powder
The next step of work was evaluating the efficiency of using cactus powder as adsorbent for chromium removal from tannery wastewater and studying the effects of adsorption conditions such as pH, adsorbent dosage and contact time on the percentage removal. For this purpose, samples of tannery wastewater were treated with cactus powder under different conditions (pH, adsorbent dosage, contact time), after what the concentration of chromium was determined. The efficiency of cactus powder was expressed as percentage removal of Cr ions which was calculated by equation (1) below:

Where Ci and Cf are the concentrations of the Cr ions before and after treatment with cactus, respectively.
Determination the Effect of pH on the Cr (VI) Removal
The adsorption process was performed at different values of pH in the range 2 – 10 at fixed adsorbent dosage (6.0 g /l) and contact time (1 hour). The results are presented in Figure 3.
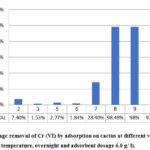 |
Figure 3: Percentage removal of Cr (VI) by adsorption on cactus |
From the figure above, it can be noted that at acidic pH the percentage removal of Cr (VI) was low, while it significantly increased in basic media to reach its maximum value at pH of 8 that was 98.48%, after that it decreased. These observations are in agreement with the mechanism proposed by [32] that is adsorption – charge neutralization and adsorption bridging mechanisms in coagulation – flocculation processes. Basing on the results obtained, the optimum value of pH was found to be 8 and this pH was used for the subsequent analyses.
In order to compare the efficiencies of cactus and calcium carbonate adsorbents for Cr (VI) removal, two separate series of chromium removal experiments were performed using cactus powder as adsorbent in one and calcium carbonate in the other within pH range 3-11. Comparison of the results shown in Figure 4 for cactus and CaCO3 enables to confirm that CaCO3 exhibited relatively high percentage removal under both acidic and basic pH values, while the highest percentage removal of chromium using cactus was obtained at basic pH values. The percentage removal using cactus was higher than that of CaCO3 at this pH range, with adsorbent dosage ( 6 g/l) five times less than that for CaCO3 (30 g/l ).
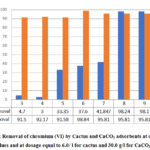 |
Figure 4: Removal of chromium (VI) by Cactus and CaCO3 adsorbents at different pH |
Determination the Effect of Adsorbent Dosage on the Cr (VI) Removal
Samples of wastewater were treated with different amounts of cactus powder at fixed pH and contact time. The amount of adsorbent ranged from 2.0 -17.8 g/l. The value of pH was adjusted at 8 which was shown to be the optimum. The results are demonstrated in Figure 5. The percent removal of Cr (VI) increased to reach the maximum value of 99% at 0.198 g/33 ml (6.0 g/l), and the subsequent increase in adsorbent dosage resulted in gradual decrease in removal. These results are consistent with previous studies [32], where they were explained by the fact that in the beginning, the increase of adsorbent dosage results in the increase of negatively charged sites of cactus polyelctrolyte and thus the positive metal ions aggregate and acquire enough size to settle, but after the maximum is reached, the increase in adsorbent dosage leads to respread of aggregated particles on the larger sorbent surface area and particle settling is disrupted and as a result the particles remain in suspension.
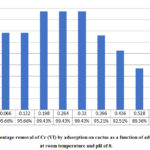 |
Figure 5: Percentage removal of Cr (VI) by adsorption on cactus |
Determination of the Effect of Contact Time on the Cr (VI) Removal
The removal of Cr (VI) using cactus with 6.0 g/l adsorbent dosage and pH of 8 at different time intervals was studied. The results are presented in Figure 6. The adsorbent achieved maximum adsorption for Cr (VI) at contact time equal to 60 min, where the percentage removal of Cr (VI) was 99.9 %. The chromium removal increased to reach the maximum value after 60 min stirring, and thereafter slightly decreased. Majority of metal ions in the wastewater are attracted to adsorption sites of Cactus with opposite charges resulting in enhanced adsorption percent by the end of the first 60 min. However, after that, progressive attachment of ions to these sites with constant amount of sorbent, leads to limited number of free adsorption sites , and as a result, the process reaches an equilibrium state [32].
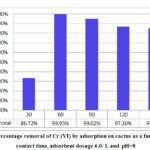 |
Figure 6: Percentage removal of Cr (VI) by adsorption on cactus |
Basing on the results obtained from the current investigation, it can be demonstrated that the optimum conditions for Cr (VI) removal using cactus powder adsorbent are the following: pH-8, adsorbent dosage-6.0 g/l and contact time -60 min. These conditions were applied for subsequent steps of analysis.
Removal of Total Chromium Using Cactus Powder
For determination of total chromium a spectrophotometric method using sulphanilic acid and N, N-dimethylaniline was used [8]. In this method, Cr (III) is initially oxidized to Cr(VI) with bromine water in basic media. Then, Cr (VI) oxidizes hydroxylamine at pH of 4 to nitrite ion that reacts with sulphanilic acid converting it to diazonium salt, which is attached to N, N-dimethylaniline in basic solution to produce methyl orange, that orange color when acidified and absorbs the visible light with wavelength of 507 nm.
Absorbance was measured for total chromium solutions of different concentrations. The obtained values of absorbance were plotted against concentration of total chromium (µg/l) to construct a calibration curve. The correlation coefficient (R2) of obtained curve was 0.999, which indicated a strong positive linear relationship between the parameters (Figure 7).
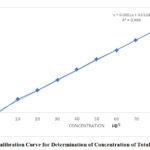 |
Figure 7: Calibration Curve for Determination of Concentration of Total Chromium. |
The concentration of total chromium in untreated tannery wastewater samples was determined using the obtained equation from calibration curve, and was found to be to 41.109 ppm, as shown in the Table below. Thereby the concentration of Cr (III) have been calculated to be equal to 33.0 ppm. This value exceeds the level declared by International environmental standards (5 ppm for Cr (III) [31]. After that, samples of wastewater were treated with cactus powder (6.0 g/l), at pH of 8, for 60 min) and then the total chromium was determined. The results are shown in the table below.
Table 1: Percentage removal of total chromium at optimum conditions (pH =8, and 6.g cactus/l).
|
|
Concentration of total chromium (ppm) |
Concentration of chromium(VI) (ppm) |
Concentration of chromium III) (ppm) |
|
Untreated wastewater |
41.1 |
8.1 |
33.0 |
|
Treated wastewater |
1.08 |
0.09 |
0.99 |
|
%Removal |
97.37 |
98.80 |
97 |
These presented results demonstrate the high efficiency of cactus powder in removing chromium ions from tannery wastewater, where the percentage removal was 97.37% for total chromium, 98.8% for toxic Cr (VI) and 97.0% for Cr (III).
In the Table 2 below, the percentage removal of Cr (VI) using various methods reported in some previous works are presented. Comparing the data shown in Table 2 with the results of this work, in addition to the previously mentioned advantages of using cactus as adsorbent for chromium ions, obviously prove the advantage of using cactus for chromium removal over other sorbents and methods used in some previous works. Therefore Cactus can be recommended as an efficient, safe and cheap adsorbent for the treatment of tannery wastewater.
Table 2: Percentage removal of Cr (VI) as reported in some previous works using different methods and sorbents
|
Method or sorbent |
% removal of Cr (VI) |
|
Chemical coagulation using aluminum sulphate and ferric chloride [3] |
74-94 |
|
Adsorption onto bentonite clay [14] |
93 |
|
Sedimentation [11] |
83.2 |
|
Natural, protonated and thermally treated Ectodermis of Opuntia [19] |
77 |
|
Natural polyelectrolyte, extracted from the cactus Opuntia ficusindica, with either: a- aluminum sulfate or b- ferric chloride [10] |
a- 83 b- 91.22 |
|
Lime/bittern coagulation and activated carbon adsorption [13] |
99.7 |
|
Polyacrylamide-grafted coconut coir pith [15] |
99.4 |
Conclusions
The results of the present work showed that Cactus acts as an excellent adsorbent for carcinogenic Cr (VI) in basic medium. The percentage removal of Cr (VI) using Cactus reached 98.8% and 97.0% for Cr (III) under the optimum conditions (pH-8, contact time-60 min and adsorbent dos age-6.0 g/l). Cactus has adsorbent capacity close to CaCO3, requiring dosage lower than CaCO3 in basic medium. Thereby Cactus can be used as low-cost alternative to other adsorbents since it readily available in nature giving an advance of it is biodegradability and formation lower sludge.
Acknowledgment
The authors of this paper would like to thank the Deanship of Graduate Studies and Scientific Research at Palestine Polytechnic University for their support in conducting this research.
Conflict of Interest
Authors of this paper have no conflict of interest.
References
- Feng, J.; Sun, Y.; Zheng, Z.; Zhang, J.; Shu, L. I. Journal of Environmental Sciences 2007, 19(12), 1409-1415.
- Ilou, I.; Souabi, S.; Digua, K. Int. J. Environm. Sci. Developm 2014, 3(5), 1706-1715.
- Song, Z.; Williams, C.; Edyvean, R. Desalination, 2004, 164(3), 249-259.
- Owlad, M.; Aroua, M. K.; Daud, W. Water, Air, and Soil Pollution 2009, 200(1-4), 59-77.
- Oliveira, H. Journal of Botany 2012, 2012, 1-8.
- Becquer, T.;, Quantin, C.; Sicot, M.; Boudot, J. P. Science of the Total Environment 2003, 301(1-3), 251-261.
- Peralta-Videa, J.R.; Lopez, M. L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The international journal of biochemistry & cell biology 2009, 41(8-9), 1665-1677.
- Chandrashekhara, K.; Gopalakrishna, B. N.; Nagaraj, P. Indian Journal of Chemical Technology 2015, 22, 78-81.
- Aregu, M.B.; Asfaw, S.L.; Khan, M.M. Environmental Systems Research 2018, 7(1), 10.
- Gomes, L.; Troiani, E. P.; Malpass, G. R.; Nozaki, J. Desalination and Water Treatment 2016, 57(22), 10181-10187.
- Song, Z.; Williams, C.; Edyvean, R. Water Research 2000, 34(7), 2171-2176.
- Mane, P. C.; Bhosle, A. B.; Jangam, C. M.; Mukate, S. V. J. Nat. Prod. Plant Resour 2011, 1(1), 75-80.
- Ayoub, G.; Hamzeh, A.; Semerjian, L. Desalination 2011, 273(2-3), 359-365.
- Tahir, S.; Naseem, R. Separation and purification Technology 2007, 53(3), 312-321.
- Unnithan, M.R.; Vinod, V.; Anirudhan, T. Industrial & engineering chemistry research 2004, 43(9), 2247-2255.
- Hashem, A.; Akasha, R. A.; Ghith, A.; Hussein, D. A. Energy Educ. Sci. Technol. 2007, 19, 69-86.
- Ravikumar, K.; Deebika, B.; Balu, K. Journal of hazardous materials 2005, 122(1-2), 75-83.
- Barrera, H.; Ureña-Núñez, F.; Bilyeu, B.; Barrera-Díaz, C. Journal of Hazardous Materials 2006, 136(3), 846-853.
- Batista, A. M.; Mustafa, A. F.; McAllister, T.; Wang, Y.; Soita, H.; McKinnon, J. J. Journal of the Science of Food and Agriculture 2003, 83(5), 440-445.
- Rodríguez-Felix, A.; Cantwell, M. Plant Foods for Human Nutrition 1988, 38(1), 83-93.
- Malainine, M. E.; Dufresne, A.; Dupeyre, D.; Vignon, M. R.; & Mahrouz, M. Zeitschrift für Naturforschung C 2003, 58(11-12), 812-816.
- Mohamed–Yasseen, Y.; Barringer, S.A.; Splittstoesser, W.E. Journal of Arid Environments 1996, 32(3), 347-353.
- Palomino, G.; Martínez, J.; Méndez, I.; Muñoz-Urías, A.; Cepeda-Cornejo, V.; Pimienta-Barrios, E. Caryologia 2016, 69(1), 82-89.
- Stintzing, F.C.; Carle, R. Molecular nutrition & food research 2005, 49(2), 175-194.
- Gad, A.S.; Attia, M.; Ahmed, H. Journal of American Science 2010, 6, 79-87.
- Fox, D. I.; Pichler, T.; Yeh, D. H.; Alcantar, N. A. Environmental science & technology 2012, 46(8), 4553-4559.
- Barka N.; Abdennouri O.K., Makhfouk M.E. J.e Taiwan Instit. Chem. Eng. 2013, 44, 52-60.
- Kumar, R.; Barakat, M. Chemical engineering journal 2013, 226, 377-383.
- Sawalha,H.; Alsharabaty, R.; Sarsour, S.; Al-Jabari, M. Journal of Environmental Management 2019, 251, 109596-109615
- Kocurek, P. A.V. E. L.; Vaskova, H.; Kolomaznik, K. A. R. E. L.; Barinova, M. Wseas. Trans. Environ. Dev 2015, 11, 256-263.
- Jabari, M.; Aqra, F.; Shahin, S.; & Khatib, A. Chemical Speciation & Bioavailability 2009, 21(3), 185-191.
- Nharingo, T.; Zivurawa, M.; Guyo, U. International journal of environmental science and technology 2015, 12(12), 3791-3802.

This work is licensed under a Creative Commons Attribution 4.0 International License.









