Polyol Synthesis of Silver Nanoparticles and Deposition on Carbon Vulcan for 4-Nitrophenol Reduction Catalysis
Canh Minh Thang Nguyen1,3, Quoc Khanh Tran1, Vinh Tien Nguyen2*
1Faculty of Chemistry, Ho Chi Minh City University of Science, Vietnam.
2Faculty of Food and Chemical Technology, Ho Chi Minh City University of Technology and Education, Vietnam.
3Laboratory of Applied Physical Chemistry, Vietnam National University, Ho Chi Minh City, Vietnam.
Corresponding Author E-mail: tiennv@hcmute.edu.vn
DOI : http://dx.doi.org/10.13005/ojc/360119
Article Received on : 16-01-2020
Article Accepted on :
Article Published : 05 Feb 2020
Silver nanoparticles (AgNP) were synthesized and then immobilized onto Carbon Vulcan support to be used as a catalyst (AgNP/C) for 4-nitrophenol (4-NP) reduction. Polyol method was used for AgNP synthesis with glycerol-water as the solvent mixture, trisodium citrate (TSC) as the reducing agent and the capping agent, silver nitrate as the silver precursor. The optimized reaction conditions were glycerol content of 35% (w/w), temperature of 105 oC, reaction time of 120 minutes, TSC/Ag+ ratio (w/w) of 6. XRD spectrum confirmed the presence of silver nanocrystallites on the prepared catalyst and TEM images their sizes mostly lower than 150 nm. The Ag/C catalyst prepared in a mildly basic solution (pH 9) showed higher catalytic activity, as well as higher reusability, compared to that prepared in a mildly acidic solution (pH 5-7).
KEYWORDS:Carbon Vulcan; Immobilization; Polyol Method; Silver Nanoparticles; 4-Nitrophenol Reduction.
Download this article as:| Copy the following to cite this article: Nguyen C. M. T, Tran Q. K, Nguyen V.T. Polyol Synthesis of Silver Nanoparticles and Deposition on Carbon Vulcan for 4-Nitrophenol Reduction Catalysis. Orient J Chem 2020;36(1). |
| Copy the following to cite this URL: Nguyen C. M. T, Tran Q. K, Nguyen V.T. Polyol Synthesis of Silver Nanoparticles and Deposition on Carbon Vulcan for 4-Nitrophenol Reduction Catalysis. Orient J Chem 2020;36(1). Available from: https://bit.ly/2ukJGDe |
Introduction
Nitroaromatic compounds, such as nitrobenzenes and nitrophenols, are abundant and chemically stable organic pollutants in agriculture and in the industry 1. Among different strategies for the disposal of nitroaromatic compounds, reducing the nitro group to an amino group is advantageous due to its high effectiveness and the importance of the reductive products in pharmaceutical and synthetic chemistry 2. Metallic nanoparticles, such as gold, silver, copper nanoparticles, have emerged as novel effective catalysts for the reduction of nitroaromatic to amino aromatic compounds 3. However, due to their small sizes, catalyst removal or recovery from the reaction mixture is one of the major challenges toward green chemical processes. A series of silver nanoparticle (AgNP) catalysts supported on activated carbon 4-6, TiO2 7, silica 8, 9, cellulose 10, 11, zeolites 12, polymers 13-15 were synthesized and used for catalytic applications.
Carbon blacks are extensively used as an electrocatalyst support due to their high mesoporous structure, high electrical conductivity, low cost and high availability 16. Different types of carbon blacks, such as acetylene black, Black Pearl, Ketjen Black, or Vulcan XC-72, have been used as electrodes in supercapacitors or catalyst supports in electrochemical applications. Among them, Vulcan XC 72R, which is industrially manufactured by Cabot Corporation, has attracted special attention due to its compromise between adequate surface area and high electric conductivity. It has been used as a support for noble-metal catalysts in low-temperature fuel cells 17. However, few studies focus on using Carbon Vulcan as a support in non-electrochemical catalysis 18, 19.
In this study, we synthesized silver nanoparticles by a polyol method without using any hazardous chemicals and then immobilized these AgNP onto activated Carbon Vulcan. The obtained material was successfully used for the first time as a catalyst for the reduction of 4-nitrophenol as a model reduction.
Materials and Methods
Materials
All chemicals were of analytical grade and used without further purification. Sodium borohydride, sodium hydroxide were purchased from Merck Millipore (USA), silver nitrate, glycerol, nitric acid, 4-nitrophenol from Xilong (China) , trisodium citrate dihydrate from Prolabo (France) , Carbon Vulcan XC72R from Cabot (Germany).
Polyol Synthesis of AgNP
Typically, predetermined amounts of glycerol, AgNO3 (1000 ppm) solution and TSC solutions (1900 ppm) were added to a glass vial containing 6.0 g of distilled water. The mixture was thoroughly stirred and heated to 95 oC and kept for 120 minutes. The reaction kinetics was monitored by recording the absorption of the reaction mixture by a UV/VIS/NIR-V670 (JASCO, Japan) spectrophotometer from 300 to 800 nm with a scan speed of 400 nm/min and a data interval of 0.5 nm.
AgNP Synthesis and in situ Deposition on Carbon Vulcan
Carbon Vulcan was activated by refluxing 0.25 g of Carbon Vulcan in 250 mL of 7% HNO3 solution for 8h. The solid was obtained by centrifugation at 4000 rpm for 15 minutes, washed three times with distilled water and dried at 110 oC for 2 hours.
After that, 0.1 g of pretreated Carbon Vulcan was ultrasonicated in 12 g of distilled water for 30 minutes. Subsequently, 18 g of glycerol was added and ultrasonicated for 30 minutes. While continuously stirred, 2.5 g of AgNO3 solution (10 000 ppm) and 7.5 g of TSC solution (20 000 ppm) were successively added. pH of the mixture was controlled using a pH meter and NaOH (1.46 M) or HNO3 (7%) solutions. The mixture was then refluxed at 105 oC for 2 hours, centrifuge at 6000 rpm for 30 minutes. The obtained solid Ag/C was then washed for two times with distilled water, one time with absolute ethanol and then dried at 60 oC for 2 hours.
Kinetic Study of Catalytic Reduction of 4-NP by NaBH4
2.5 mg of Ag/C catalyst was added to 6.25 g of distilled water in a vial. The mixture was suspended in a sonication bath for 5 minutes. 0.1 mL of the suspension was transfered to another vial with 9.2 g of water. Subsequently, 0.1 mL of 0.05 M NaHCO3 and 0.1 mL of 5 mM 4-NP were added. After ultrasonication, 0.5 mL of 0.1 M NaBH4 was added and shaken thoroughly. The reaction kinetics was followed by recording the absorbance of the suspension 400 nm over time. All experiments were repeated at room temperature (27 oC) for 3 times. In all kinetic experiments,the initial concentration of NaBH4 was 100 times higher, than that of 4-NP concentration. Therefore, NaBH4 concentration was considered unchanged and did not affect the reaction rate during the reaction.
Results and Discussion
Polyol Synthesis of AgNP in Solution
During heating the AgNO3 solution with glycerol, the solution turned from colorless to pale-yellow with maximum absorbance ranging from 408 to 416 nm due to the surface plasmon resonance (SPR) of silver nanoparticles 20. In order to optimize the synthesis conditions, the effects of glycerol concentration, reaction time, temperature, TSC concentration were investigated. Many studies proved that higher values of SPR absorbance of the solution correspond to a higher concentration of AgNP. Therefore, we used the maximum absorbance of the solution at SRP wavelengths (around 410 nm) as the criteria for the optimum condition 21. Preliminary experiments showed that the synthesis of AgNP still went on after 30 minutes, and only stopped after 120 minutes. Therefore, 30 minutes were chosen as the reaction time when optimizing the other factors.
Influence of Glycerol Concentration
In polyol synthesis of metallic nanoparticles, the polyol can play the role of a cosolvent, a reducing agent, or/and a capping agent 22. Therefore, its amount in the reaction medium plays an important role. Preliminary result (Figure 1a) showed that raising the glycerol content up to 45% increased the amount of AgNP. However, when moving from 30% glycerol (absorbance = 1.37) to 45% glycerol (absorbance = 1.77), the increase of absorbance was relatively small. Moreover, high content of glycerol would increase the viscosity of the solution, which may negatively affect the deposition process of AgNP onto Carbon Vulcan. We decided not to test higher concentrations of glycerol, but to investigate more closely its range from 25% to 40% (Figure 1b). The results showed that 35% of glycerol produced the highest amount of AgNP, and thus was chosen as the optimum glycerol concentration for AgNP/C synthesis.
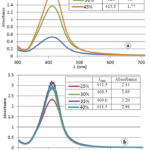 |
Figure 1: Influence of glycerol concentration on absorption |
Influence of Temperature
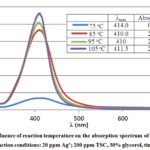 |
Figure 2: Influence of reaction temperature on the absorption spectrum |
Temperature is one of the most important factors for any chemical reaction. Figure 2 shows that at 75 oC, the reaction extent was limited. However, increasing the reaction temperature up to 105 oC produced substantially more AgNP. Obviously, at 30 minutes the polyol reaction still took place, so increasing the temperature would speed up the reaction and produce higher amount of AgNP. Further increase the temperature would make the solution boil, which requires a more complex synthetic system with a condenser to control the concentrations of the reaction components. Therefore, in the investigated range of reaction temperatures, 105 oC was chosen as the appropriate temperature for AgNP/C synthesis.
Influence of TSC Concentration
It is well known that TSC plays the dual role of a reducing agent and a capping agent during the polyol synthesis of AgNP 22. When TSC was not added to the reaction mixture, the characteristic peak of AgNP at around 410 nm did not appear. This fact demonstrated the crucial role of TSC in AgNP formation. When the TSC concentration was 40 ppm (2 times of [Ag+]), AgNP began to form, but at a low extent. When the TSC concentration was 120 ppm (6 times of [Ag+]) and higher, AgNP concentration almost reached a maximum. Therefore, TSC concentration of six times that of silver ions was chosen as optimized value for AgNP/C catalyst.
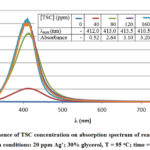 |
Figure 3: Influence of TSC concentration on absorption spectrum |
Characterization of AgNP/C
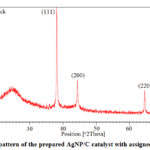 |
Figure 4: XRD pattern of the prepared AgNP/C catalyst with assigned silver peaks. |
XRD spectrum of a synthesized AgNP/C sample (Figure 4) was compared with the standard spectrum of the face-centered cubic (fcc) structure of bulk silver (JCPDS file No. 04-0783). The spectrum shows four characteristic peaks of metallic silver at 2θ values of 38.2o (111), 44.3o (200), 64.5o (220) and 77.5o (311), what clearly shows that the as-prepared material contained ffc crystallite silver together with the mostly amorphous Carbon Vulcan. Based on the Scherrer equation, the mean size of the silver crystallites was of ca.30 nm.
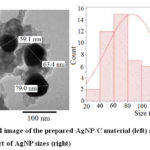 |
Figure 5: TEM image of the prepared AgNP/C material (left) and frequency chart |
Analysis of TEM images of the AgNP/C prepared in different pH showed that AgNP (Z=48) particles appeared as black figures, while the Carbon Vulcan (Z=6) support as grey clusters. About 90% of the AgNP had sizes lower than 150 nm, with a mean value of about 70 nm. This value is at the same order with the mean crystallite size obtained by the XRD spectrum above and the Scherrer equation.
Catalytic activity on 4-NP reduction
Figure 6 shows the change of absorbance (λ = 400 nm) of 4-NP ate ions in the solution after adding a constant amount (2.5 mg) of the synthesized AgNP/C catalyst. After an initial induction period due to the reduction of dissolved oxygen by NaBH4 23, the absorbance of 4-NP decreased for 200-300 seconds. Because NaBH4 is in great excess in the reaction, its concentration can be considered constant throughout the reaction. Therefore, pseudo-first-order kinetics can be applied with respect to the concentration of 4-NP 3.
The time periods corresponding to from 60% to 40% of initial absorbance Ao were chosen for calculation of the apparent rate constants. This choice of intermediate time periods was based on the assumption that, in the proximity of the beginning (A > 60% Ao) or the end (A < 40% Ao) of the reaction, the overall reaction kinetics is more heavily influenced by the diffusion of reactants/products to/from the catalyst surface. The apparent rate constants show that AgNP/C catalysts prepared under basic conditions had higher catalytic activity than those prepared under acidic conditions.
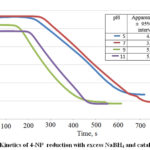 |
Figure 6: Kinetics of 4-NP reduction with excess NaBH4 and catalyst AgNP/C |
The reusability of the prepared AgNP/C catalyst was evaluated by adding more 4-NP into the reaction mixture every time the reaction has stopped . After each cycle of usage, the catalytic activity of all prepared catalysts samples decreased, as demonstrated by the increase of reaction half-lives (Figure 7). This is possibly due to the adsorption of reaction products onto the AgNP surface over time, which reduced the free active centers of the catalyst. Moreover, from the slope of the half-life vs reaction cycle, we can compare the stability of the prepared catalysts toward reuse. It can be seen that the AgNP/C catalyst prepared at mildly alkaline medium (pH 9) was the most stable, while that prepared in acidic (pH 5) and neutral medium (pH 7).
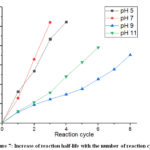 |
Figure 7: Increase of reaction half-life with the number of reaction cycles |
Conclusions
In this study, AgNP were synthesized by polyol method and then immobilized onto Carbon Vulcan support to be used as a catalyst for 4-NP reduction. The optimum conditions for AgNP synthesis suspension were determined and applied to prepare AgNP/C catalyst. AgNP/C catalysts prepared in mildly basic solutions showed higher catalytic activity, as well as higher reusability, compared to those prepared in mildly acidic solutions.
Acknowledgement
The authors sincerely acknowledge Ho Chi Minh City University of Technology and Education for the financial support and Vietnam National University, Ho Chi Minh City University of Science for the laboratory and equipment supports to complete this study.
Conflict of Interests
The authors declare no conflict of interest.
References
- Tiwari, J.; Tarale, P.; Sivanesan, S.; Bafana, A., Sci. Pollut. Res. 2019, 1-18.
- Muniz-Miranda, M., Catal. B-Environ. 2014, 146, 147-150.
CrossRef - Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M.; Fenger, R.; Rademann, K.; Jaquet, B.; Zaccone, A., . Phys. Chem. C 2014, 118, 18618-18625.
CrossRef - Sudhakar, P.; Soni, H., Environ. Chem. Eng. 2018, 6, 28-36.
CrossRef - Rengga, W. D. P.; Chafidz, A.; Sudibandriyo, M.; Nasikin, M.; Abasaeed, A. E., Environ. Chem. Eng. 2017, 5, 1657-1665.
CrossRef - Altintig, E.; Arabaci, G.; Altundag, H., Coat. Technol. 2016, 304, 63-67.
CrossRef - Saran, S.; Manjari, G.; Devipriya, S. P., Clean. Prod. 2018, 177, 134-143.
CrossRef - Yan, Z.; Fu, L.; Zuo, X.; Yang, H., Appl Catal B-Environ 2018, 226, 23-30.
CrossRef - Khrizanforov, M. N.; Fedorenko, S. V.; Mustafina, A. R.; Kholin, K. V.; Nizameev, I. R.; Strekalova, S. O.; Grinenko, V. V.; Gryaznova, T. V.; Zairov, R. R.; Mazzaro, R., Dalton Transactions 2018, 47, 9608-9616.
CrossRef - Han, Y.; Wu, X.; Zhang, X.; Zhou, Z.; Lu, C., ACS Sustain. Chem. Eng. 2016, 4, 6322-6331.
CrossRef - Goswami, M.; Baruah, D.; Das, A. M., New J. Chem. 2018, 42, 10868-10878.
CrossRef - Dutta, P.; Wang, B., Chem. Rev. 2019, 383, 1-29.
CrossRef - Patra, S.; Naik, A. N.; Pandey, A. K.; Sen, D.; Mazumder, S.; Goswami, A., Catal. A-Gen. 2016, 524, 214-222.
CrossRef - Kamal, T.; Ahmad, I.; Khan, S. B.; Asiri, A. M., Polym. 2017, 157, 294-302.
CrossRef - Ali, N.; Kamal, T.; Ul-Islam, M.; Khan, A.; Shah, S. J.; Zada, A., J. Biol. Macromol. 2018, 111, 832-838.
CrossRef - Ma, Y.; Wang, H.; Ji, S.; Goh, J.; Feng, H.; Wang, R., Acta 2014, 133, 391-398.
CrossRef - Shuihua, T.; Gongquan, S.; Jing, Q.; Shiguo, S.; Junsong, G.; Qin, X.; Haarberg, G. M., Chinese J Catal. 2010, 31, 12-17.
CrossRef - Xia, J.; He, G.; Zhang, L.; Sun, X.; Wang, X., Catal. B-Environ. 2016, 180, 408-415.
CrossRef - Qin, L.; Yi, H.; Zeng, G.; Lai, C.; Huang, D.; Xu, P.; Fu, Y.; He, J.; Li, B.; Zhang, C., Hazard. Mater. 2019, 380, 120864.
CrossRef - Kastner, C.; Thunemann, A. F., Langmuir 2016, 32, 7383-7391.
CrossRef - Zook, J. M.; Long, S. E.; Cleveland, D.; Geronimo, C. L. A.; MacCuspie, R. I., Anal. Bioanal. Chem 2011, 401, 1993.
CrossRef - Khodashenas, B.; Ghorbani, H. R., J. Chem. 2019, 12, 1823-1838.
CrossRef - Menumerov, E.; Hughes, R. A.; Neretina, S., Nano Lett. 2016, 16, 7791-7797.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









