Immune Recognition of Closed and Open Lactam Rings and Their Influence on Immunoassays of Ampicillin Antibiotics
Nadezhda S. Komova, Anna N. Berlina , Anatoly V. Zherdev and Boris B. Dzantiev*
A.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Leninsky prospect 33, 119071 Moscow, Russia
Corresponding Author E-mail: dzantiev@inbi.ras.ru
DOI : http://dx.doi.org/10.13005/ojc/360103
Article Received on : 15 Nov 2019
Article Accepted on : 07 Jan 2020
Article Published : 20 Jan 2020
Open-ring derivatives of beta-lactams do not inhibit bacterial growth but retain several biological activities. In this regard, methods of antibiotic control should be characterized by specificity to such derivatives. The objective of this study was to evaluate commercially available immunoreagents for the detection of beta-lactams from the point of view of their selectivity with respect to the open-ring derivatives of lactams. The enzyme-linked immunosorbent assays (ELISA) was used for this purpose. Ampicillin was hydrolyzed by acid and alkaline treatments, and opening of the lactam ring was confirmed spectrophotometrically. It was shown that either selective detection of only native ampicillin or integrated detection of both its states could be realized depending on the immunoreagents. The detection limit for native ampicillin for both assays was 10 ng/ml, whereas cross-reactivity to the derivatives with an open ring was <0.01% for the first case and 61.5–64.2% for the second case. The established variability is critical in interpreting the data of immunodetection and should be controlled for grounded evaluation of pharmaceuticals and foods.
KEYWORDS:Beta-Lactams; Destruction of Antibiotics; ELISA; Selectivity of Assays; Safety of Foods; Quality of Pharmaceuticals
Download this article as:| Copy the following to cite this article: Komova N. S, Berlina A.N, Zherdev A. V, Dzantiev B. B. Immune Recognition of Closed and Open Lactam Rings and Their Influence on Immunoassays of Ampicillin Antibiotics . Orient J Chem 2020;36(1). |
| Copy the following to cite this URL: Komova N. S, Berlina A.N, Zherdev A. V, Dzantiev B. B. Immune Recognition of Closed and Open Lactam Rings and Their Influence on Immunoassays of Ampicillin Antibiotics . Orient J Chem 2020;36(1). Available from: https://bit.ly/2uhOYyO |
Introduction
Beta-lactam antibiotics play an important role in the treatment of human infections. [1] The first beta-lactam antibiotic applied for human diseases treatment was Penicillin G in 1940s but in 1944 the information about destruction of the lactam ring by resistant S. aureus was published.[2] Their wide use as tools to enhance growth and yield in agriculture makes them present in foodstuffs (causing toxicity, allergenicity, and induction of microorganisms’ resistance)[3]. Therefore, the control of antibiotics is important for medicine and food safety.[4, 5] Among the methods used for this purpose [6, 7], immunochemical methods are of particular interest because of their simplicity, lack of need for sophisticated equipment, and ability to obtain quick results. [8, 9] The immune methods developed and recommended for mass control are characterized in detail by their specificity to other natural and artificial compounds that could potentially be in the tested samples. However, revealing partially degraded derivatives of antibiotics using antibodies remains unexplored. This question is especially important for beta-lactam antibiotics being inactivated by the destruction of their lactam ring either with significant changes in pH [10] or under the action of special enzymes—lactamases. [11] The antibiotic resistance of pathogenic bacteria, which has become one of the main problems for antibiotic therapy in recent years, in many cases is because of the lactamase activity of such bacteria. [12] Note that lactam derivatives with open rings, while losing their toxicity to bacteria, retain many other biological properties (for example, allergenicity). Accordingly, when assessing the effectiveness of antibiotic therapy, it is critical to identify precisely the native lactam molecules, while the contamination of food products in accordance with current regulations should consider both native antibiotic molecules and their derivatives.
The objective of this study was to evaluate commercially available immunoreagents for the detection of beta-lactams from the point of view of their selectivity with respect to the open-ring derivatives of lactams. We were interested in the types of analyses that can be implemented using different completions of analytical systems and what methodology for testing the specificity of the analysis will adequately evaluate its informational output. In the implemented experiments, ampicillin was considered one of the most widely used beta-lactam antibiotics.[13]. To obtain standard preparations of ampicillin derivatives with an open ring, acid and alkaline hydrolysis was used, the effectiveness of which was shown in previously published works. [14, 15]
Materials and Methods
Chemicals and reactants
Ampicillin (AMP) sodium salt was purchased from Sigma-Aldrich (St. Louis, US). Mouse monoclonal antibody (#1) against AMP and ampicillin-bovine serum albumin (BSA) conjugate (#2) were purchased from EximBio (Shanghai, China). Monoclonal antibody against AMP (#3) and penicillin-BSA conjugate (#4) were purchased from Maine Biotechnology Services (Portland, US) and DCN (Carlsbad, US), respectively. Peroxidase-labelled rabbit anti-mouse polyclonal antibodies were acquired from Imtek (Moscow, Russia), and 3,3′,5,5′-tetramethylbenzidine (TMB) and surfactant Triton X-100 were acquired from Panreac Química (Barcelona, Spain). Anti-species antibodies against mouse IgG labeled with horseradish peroxidase were obtained from the N.F. Gamaleya Institute of Epidemiology and Microbiology (Moscow, Russia). Albumin from bovine sources (BSA) was obtained from Boval Biosolutions (Cleburne, US). All other reagents were of analytical-grade purity or greater.
A set of 96-well transparent flat-bottom polystyrene microplates 9018 was procured from Costar (New York, US). Deionized water (18 MΩ·cm at 25°C; Simplicity Millipore, Billerica, US) was used to prepare all solutions.
Hydrolysis of ampicillin
For alkaline hydrolysis,10 mg of ampicillin sodium salt was dissolved in 1 mL of 0.2 M NaOH and incubated for 2 hours at 37°C [14]. For acid hydrolysis, 10 mg of ampicillin sodium salt was dissolved in 1 mL of 5 M HCl and incubated for 2 hours at room temperature [15]. The changes in structure of ampicillin were confirmed by absorption spectra that were recorded using spectrophotometer Shimadzu 1601 (Kyoto, Japan). Thereafter, ampicillin-hydrolyzed solutions were adjusted to pH 7 for possible use for immune detection. The obtained preparations were stable and stored at 4°C. To obtain reproducible results, the preparations were stored in a refrigerator and analyzed in three repetitions for 5 consecutive days to assess the stability and standard deviations of the results of analysis.
ELISA. A solution of hapten–BSA conjugate (2 µg/mL in 50 mM phosphate buffer saline, pH 7.4 [PBS]) was introduced into the wells of microplates, 100 µl/well. After overnight incubation at 4°C or a 2-hour incubation at 37°C, the microplate was washed three times using PBST (PBS with 0.05% Triton X-100) with 150 µl/well per wash cycle. After that, 50 µL of ampicillin solution at concentration range of 5 µg/mL–80 pg/mL and 50 µL of monoclonal antibodies against ampicillin at final concentrations of 21 ng/mL and 7 ng/mL (for reagents #1 and #3, respectively, both in PBST) were added to the wells and incubated for one hour at 37°C. After 3 cycles of washing with PBST, anti-species antibody labelled with peroxidase (dilution 1:5,000 of commercial preparation in PBST) was added at 100 µL/well and incubated for one hour at 37ºC. Then, the microplate wells were washed 3 times with PBST. Next, 100 µL of substrate solution (0.4 мМ TMB, 3 mM H2O2) was added into each well, and after color developed, the reaction was stopped by the addition of 50 µL of 1 M H2SO4. Finally, absorbance at 450 nm was recorded using the Zenyth 3100 microplate reader (Anthos Labtec Instruments, Salzburg, Austria).
The completion I for specific detection of native ampicillin included reactants #1 and #2. The completion II for integrated detection of native and hydrolyzed ampicillin included reactants #3 and #4.
Processing of the Data
Calibration curves were constructed by plotting the optical density at 450 nm (A450) as a function of the known ampicillin concentration using Origin 7.5 (Origin Lab, Northampton, US) software. The limit of detection was calculated as the concentration that generated a signal three times higher than the standard deviation of the background signal (signal in absence of ampicillin).
Results
The products of acid and alkaline treatments of ampicillin were obtained. The recorded absorbance spectra of native, acid-, and alkaline-hydrolyzed ampicillin are shown in Fig. 1. They demonstrated known specific peaks: 330 nm for native ampicillin, 370 nm after acid hydrolysis, and an additional peak at 470 nm after alkaline hydrolysis.
Three obtained preparations were used to test different completions of immunoreactants for competitive ELISA. After consideration of possible reactant combinations, two variants of ELISA, differing in specificity, were proposed.
The completion I provides specific detection of native ampicillin with the detection limit 1.7 ng/mL. Cross-reactivities for acid- and alkaline-hydrolytic products were <0.01%. Some concentration-independent decrease of binding values for this preparation could be associated with the influence of hydrolytic media on immune interactions.
The completion II provides group detection of both native and derived ampicillin (see Fig. 3). The reached detection limit for ampicillin was 9.2 ng/mL. After alkaline hydrolysis, 64.2% of immune reactivity was stored (Fig. 3a), and after acid hydrolysis, 61.5% (Fig. 3b). The given immune reactants are tolerant to the studies of treatment, and the binding values without ampicillin in the samples are practically the same.
Discussion
In the present study, we evaluated the possibility of ampicillin detection using commercially available immunoreagents and estimated the selectivity of the proposed method to the open-ring derivatives of lactams.
Preparations of open-ring derivatives of ampicillin were obtained by acid and alkaline hydrolysis using the previously described techniques [14, 15]. To confirm the changes in the structure of ampicillin molecule after hydrolysis, the absorbance spectra of products were measured (Fig. 1). The bathochromic shifts of maximum on 41 nm (from 330 nm to 371 nm) for acid hydrolyzed products and 70 nm (from 330 nm to 400 nm, with additional peak at 481 nm) for alkaline hydrolyzed ampicillin were observed. Jones et al. [16] observed the shift in 65 nm from 345 nm to 410 nm when studied the hydrolysis of CENTA compound by beta-lactamase. CENTA is synthesized chromogenic cephalosporin reagent, which is beta-lactamase-labile, and changes color after the hydrolysis of the beta-lactam ring. Makena et al. [17] demonstrated the bathochromic shift in 59 nm (from 347 nm to 406 nm) for hydrolyzed CENTA compound compared to native preparation. Thus, the effects observed in this work are similar to the data of predecessors. This fact confirms the complete destruction of the lactam ring for the both treatments. It should be noted that hydrolysis products significantly depend upon the kind of treatment applied. As follows from the existing data [10], at pH 2 the product of lactam hydration with acid is obtained, the low-speed transformation at pH 7 leads to obtaining an epimer of ampicillin, and the degradation at pH > 9 leads to formation of two stereoisomers with open ring (Fig. 4). The study on determination of minimal inhibition concentration of beta-lactam antibiotics at different storage conditions on bacterial strains showed strong dependence on temperature as well as pH [18]. Thus, solutions of antibiotics of this class are stable at -70°С preferably [18] to keep antibacterial properties. Varying pH of ampicillin solutions showed that antibiotic is much less stable at alkali pH [19], but relatively stable at acidic which allows to take it as medicine to treat bacterial infections orally. From these points of view, alkaline hydrolysis is more preferable to disrupt lactam ring, but we used two techniques to compare the results.
The obtained preparations were used to test specificity of immunoassays with different combinations of immune reactants. We found that the given specificity may differ significantly (see Figs. 2, 3), causing the possibility of incorrect conclusions in the testing efficiency of pharmaceuticals (where only levels of active antibiotics with closed lactam rings are diagnostically significant) and in food testing (where the sum of all derivatives should be controlled). As seen from Figure 2, the products obtained by the different ways (acidic and alkaline hydrolysis) led no to decrease in binding to antibody which reflects a lack of recognition by immunoreagentsreagents in this experiment (curves A and B of Fig. 2). The main result is that the hydrolyzed product can not be an effective competitor in immunoassay because it is not recognized with the antibody in this situation (dash lines, Fig. 2) compared to native ampicillin (solid lines, Fig. 2). Competitive curves on Fig. 3, contrary to Fig. 2 have a slight difference in the working ranges of the determined concentrations for the two hydrolytic products, which reflects the different affinity of their binding to these antibodies. But it does not prevent detection using an existing set of immunoreagents. The obtained preparations of hydrolyzed derivatives of ampicillin are stable under storage (in solved form at 4°C), and, therefore, may be recommended as additional tested samples for characterization of immunoassays used in future studies.
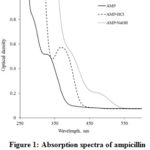 |
Figure 1: Absorption spectra of ampicillin and acid/basic-hydrolyzed ampicillin |
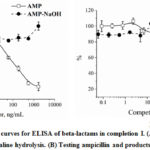 |
Figure 2: Competitive curves for ELISA of beta-lactams in completion I. |
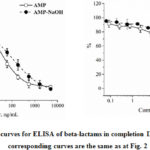 |
Figure 3: Competitive curves for ELISA of beta-lactams in completion II. Click here to View Figure |
![Figure 4: Methods of ampicillin hydrolysis at different pH (based on [10], with modifications)](http://www.orientjchem.org/wp-content/uploads/2020/01/Vol36No1_Imm_Nad_fig4-150x150.jpg) |
Figure 4: Methods of ampicillin hydrolysis at different pH (based on [10], with modifications) |
Acknowledgements
This study was financially supported by the Ministry of Science and Higher Education of the Russian Federation (agreement 14.613.21.0061 from July 17, 2017; the unique identification number of the project is RFMEFI61317X0061).
Conflicts of Interests
The authors declare that they have no conflict of interest.
References
1. Pruden, A., M. Arabi,H. N. Storteboom. Environmental Science & Technology, 2012. 46(21): 11541-11549.
2. Kirby, W. M. M. Science, 1944. 99(2579): 452-453.
3. Goldman, E. Human and Ecological Risk Assessment: An International Journal, 2004. 10(1): 121-134.
4. Byzova, N. A., E. A. Zvereva, A. V. Zherdev,B. B. Dzantiev. Applied Biochemistry and Microbiology, 2011. 47(6): 627.
5. Berlina, A. N., A. V. Bartosh, A. V. Zherdev, C. Xu,B. B. Dzantiev. Antibiotics (Basel, Switzerland), 2018. 7(4): 99.
6. Carlier, M., V. Stove, S. C. Wallis, J. J. De Waele, A. G. Verstraete, J. Lipman,J. A. Roberts. International Journal of Antimicrobial Agents, 2015. 46(4): 367-375.
7. Kantiani, L., M. Farré, D. Barceló,D. Barceló. TrAC Trends in Analytical Chemistry, 2009. 28(6): 729-744.
8. Li, Y.-F., Y.-M. Sun, R. C. Beier, H.-T. Lei, S. Gee, B. D. Hammock, H. Wang, Z. Wang, X. Sun, Y.-D. Shen, J.-Y. Yang,Z.-L. Xu. TrAC Trends in Analytical Chemistry, 2017. 88: 25-40.
9. Taranova, N., A. Berlina, A. Zherdev,B. Dzantiev. Biosensors and Bioelectronics, 2015. 63: 255-261.
10. Mitchell, S. M., J. L. Ullman, A. L. Teel,R. J. Watts. Science of The Total Environment, 2014. 466-467: 547-555.
11. Livermore, D. M. Journal of Antimicrobial Chemotherapy, 1993. 31(suppl_A): 9-21.
12. Blanca, M., M. J. Torres, J. J. García, A. Romano, C. Mayorga, E. de Ramon, J. M. Vega, A. Miranda,C. Juarez. Journal of Allergy and Clinical Immunology, 1999. 103(5): 918-924.
13. Kong, K.-F., L. Schneper,K. Mathee. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica, 2010. 118(1): 1-36.
14. Sawai, T., I. Takahashi,S. Yamagishi. Antimicrobial agents and chemotherapy, 1978. 13(6): 910-913.
15. Forró, E.,F. Fülöp. Organic Letters, 2003. 5(8): 1209-1212.
16. Jones, R. N., H. W. Wilson, W. J. Novick, Jr., A. L. Barry,C. Thornsberry. J Clin Microbiol, 1982. 15(5): 954-958.
17. Makena, A., S. S. van Berkel, C. Lejeune, R. J. Owens, A. Verma, R. Salimraj, J. Spencer, J. Brem,C. J. Schofield. ChemMedChem, 2013. 8(12): 1923-1929.
18. Nickolai, D. J., C. J. Lammel, B. A. Byford, J. H. Morris, E. B. Kaplan, W. K. Hadley,G. F. Brooks. Journal of Clinical Microbiology, 1985. 21(3): 366-370.
19. Robinson-Fuentes, V. A., T. M. Jefferies,S. K. Branch. Journal of Pharmacy and Pharmacology, 1997. 49(9): 843-851.

This work is licensed under a Creative Commons Attribution 4.0 International License.









