Evaluation of Antioxidant Activity and Membrane Stability of three Unani Medicines Preparation in Bangladesh
Sazaul Morshed Sazib1, Sadia Afroz2, Tariqul Islam3, Abdullah Al Ragib2*,4, Muhammad Zukaul Islam5 and Abdullah Al Mahmud6
1Department of Pharmacy, Noakhali Science and Technology University, Noakhali-3814, Bangladesh
2Department of Applied Chemistry and Chemical Engineering, Noakhali Science and Technology University, Noakhali-3814, Bangladesh
3Department of Pharmaceutical Technology, International Islamic University Malaysia, 25200 Kuantan, Pahang, Malaysia
4School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, China
5Department of Chemistry, Bangladesh University of Engineering and Technology, Dhaka-1000, Bangladesh
6Department of Pharmacy, Manarat International University, Dhaka-1212, Bangladesh
Corresponding Author E-mail: ragibnstu@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360117
Article Received on : 01 Jan 2020
Article Accepted on : 15 Feb 2020
Article Published : 19 Feb 2020
A comparative pharmacological study of three different unani medicines are performed, trade named Safi, Musaffi and Hemosaf used for blood purification. In which, different concentration of the syrups were evaluated for its possible membrane stabilizing and antioxidant activity. Gallic acid and ascorbic acid were used as a standard for antioxidant activity. Acetyl salicylic acid (ASA) (0.10 mg/ml) was also used as standard drug for membrane stability. The amount of total phenolic content for Safi, Musaffi and Hemosaf was subsequently 542.2 mg, 444.27 mg and 432.20 mg of GAE/mg of sample solution. DPPH radical scavenging activities of three Samples were increased with the increasing concentration. Maximum inhibition (radical scavenging) 85.96% for Hemosaf was observed at 100 µg/ml concentration with IC50 of 1.91 µg/ml compared to standard (ascorbic acid) inhibition 95.86% with IC50 of 1.93 µg/ml. Among all the syrup fractions, at 8.0 mg/ml concentration maximum effect with a value of 50.24%, 47.70% and 34.85% hindrance of hemolysis of Red Blood Cell (RBC) caused by hypotonic solution of Safi, Musaffi and Hemosaf respectively as compared to 67.01% showed by standard acetyl salicylic acid (ASA). Finally, it might be a source of antioxidant activity and possess good membrane stability effects.
KEYWORDS:Antioxidant Activity; Membrane Stabilization; Radical Scavenger; Unani Medicine
Download this article as:| Copy the following to cite this article: Sazib S. M, Afroz S, Islam T, Al Ragib A, Islam M. Z, Al Mahmud A. Evaluation of Antioxidant Activity and Membrane Stability of three Unani Medicines Preparation in Bangladesh. Orient J Chem 2020;36(1). |
| Copy the following to cite this URL: Sazib S. M, Afroz S, Islam T, Al Ragib A, Islam M. Z, Al Mahmud A. Evaluation of Antioxidant Activity and Membrane Stability of three Unani Medicines Preparation in Bangladesh. Orient J Chem 2020;36(1). Available from: https://bit.ly/39ICekr |
Introduction
Thousands of years ago from the history of human evaluation the use of medicinal plants by human beings to treat diverse diseases1. From the early stage of human civilization, natural products and medicinal plants which are used by indigenous people as a traditional medicine are of great importance to discover a number of modern drugs2. To early humans the use of natural and biomedicines have presented a great challenge and later by the development of outstanding therapeutic tools and technology they were able to develop knowledge about edible materials, phytomedicine, biomedicine or natural medicines3. Traditional or complementary medicine gives advantages not only in the field of exploring pharmacological, pharmacokinetic and toxicological features over other forms of medicine but also gives favorable position in such areas of discovery of drug candidates and examining drug-like activity2,4. In addition, serious side-effects and high cost of number of modern medicines is a great solicitous in public health and for this today people like to depend on medicinal plants as a source for discovery of newer unani or phyto-based drugs with low cost and less side-effects5. Scientific report shows that not less than 80% of the world’s population still depends on phytotherapy and 64% of the world populations in both urban and rural areas totally tend to use traditional medicinal plants to protect and cure their health from various dangerous diseases6,7. Chemical constituents and medicinal uses of 449 medicinal plants growing and available in South Asia especially Bangladesh are listed. Because of the availability of these indigenous medicinal plants they are extensively used in the preparation of unani and ayurvedic medicines in South Asia like Bangladesh, India, Nepal, Bhutan etc. But the fact is that almost all of them are lacking proper scientific evaluation of their therapeutic uses. So, time demands, standard pharmacological protocol and phytochemical analysis to establish scientific evidence of the efficiency of these preparations in pharmacological use8.
Materials and Methods
Reagents, Chemicals and Instruments
Folin-Ciocaltu reagent, gallic acid, sodium carbonate (7.5 % w/v), distilled water, syrup solution ( 3 unani samples), DPPH (1,1-diphenyl-2-picrylhydrazyl), standard acetyl salicylic acid (ASA), human red blood cell (RBC), anticoagulant EDTA, phosphate buffer (pH 7), sodium chloride, isotonic solution, glass ware, stirring bar, UV spectrophotometer, weighing machine etc. All chemicals are purchased from Merk, Germany.
Antioxidant Activity
Total Phenolic Content
To determine the total phenolic content of all the unani preparations Folin-Ciocalteu reagent was used9. Folin-Ciocalteu reagent produced complex blue color which is a combination of phosphomolybdate and phosphotungstate reduced the polyphenols containing samples10. A gallic acid calibration curve is used to estimate the phenolic concentration of extracts sample. So it is important to prepare a calibration curve and for this firstly we need to prepare 0.5 ml aliquots. The concentration should be maintained within a range which is 100 μg/ml to 6.25 μg/ml gallic acid solutions and then 2.5 ml of diluted (at a ratio of 1:10 with water) Folin-Ciocalteu reagent were mixed. Then, 7.5% (w/v) sodium carbonate (2.0 ml) was added to neutralize the mixture. After that 20 minutes incubation is maintained for color progression at 25°C and total phenolic content of the sample solution was estimated at 760 nm in contradiction of reagent blank sample. The total phenolic contents of extract sample were expressed as mg of gallic acid equivalent weight (GAE) and absorbance vs. concentration curve was constructed which showed a liner relationship.
Assay for DPPH Activity
The stable DPPH radical-scavenging activity was measured by follow the Gupta’s design with slightly modification11,12. In this method stock solutions (1mg/ml) of each sample were prepared in respective and universal solvent (water). To prepare stock solutions serial dilutions were carried out and having the range of 100 µg/ml to 12.5 µg/ml concentration. In this investigation, sample solution (2ml) at various concentration in which 2 ml of 0.2 mM methanolic DPPH was added and in the mean time it was stirred strenuously (15 seconds). After that at room temperature the solution must be kept at dark place because of reaction condition in which the solutions were allowed to stand approximately 30 min. After that absorbance was performed at 517 nm against a reagent blank13. From the graph the IC50 value of each sample was estimated by non-linear regression. Ascorbic acid was used as positive control standard.
Membrane Stabilizing
In vitro membrane stabilizing screening of was performed by following the methods developed by Sikder et al.14.
Hypotonic Solution- Induced Haemolysis
Suspension of RBC used as stock test sample that was collected from male human under standard condition. Then, the hypotonic solution were used to perform the experiment. In addition, isotonic solution (154 mM NaCl) and sodium phosphate buffer (pH 7.4 & 10 mM) were used to wash the blood three times by centrifugation in which time limit was 10 minutes at 3000 g (RPM). After that RBC suspension was finally collected as a stock and EDTA was used for the prevention of clotting. So for the evaluation of membrane stabilizing, the stock solution, 5 ml of hypotonic solution (50 mM NaCl) in 10 mM sodium phosphate buffer saline (pH 7.4), 3 different unani medicine Safi, Musaffi and Hemosaf (1.0 mg/mL) and ASA (Acetyl Salicylic Acid) was used. The Acetyl Salicylic Acid (ASA) was used as a reference standard (0.1 mg/mL). Then incubation was done for the duration of 10 minutes at room temperature and also at the same time centrifugation was also performed for 10 minutes (RPM 300). UV spectrophotometer (Shimadzu) measured the absorbance of the unani sample at 540 nm. Inhibition percentage of membrane stabilization was determined by standard protocol and shown in table 3 15,16.
Experimental Results
Total phenolic content determination and DPPH radical scavenging activity of different unani samples for antioxidant activity identification and effect of extract of Safi, Musaffi and Hemosaf on hypotonic solution-induced haemolysis of erythrocyte membrane for Membrane Stabilizing activity identification have been described.
Antioxidant Activity
Total Phenolic Content Determination
In this method, we depend and continue our investigation based on the absorbance values of different sample solutions by the colorimetric analysis in which the total phenolic content of different samples was determined and gallic acid equivalents was used to compare the sample as a standard solution.
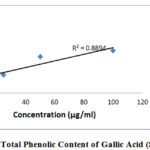 |
Figure 1: Total Phenolic Content of Gallic Acid (Standard) |
Table 1: Determination of Total Phenolic Content of 3 Unani syrups (3 samples prepared from one syrup)
|
Syrup Name |
Sample preparation |
Absorbance |
Average Absorbance |
Total Phenolic Content (mg of GAE / mg) of Sample |
|
Safi |
01 |
1.898 |
1.68 |
542.20 |
|
02 |
1.732 |
|||
|
03 |
1.411 |
|||
|
Musaffi |
01 |
1.596 |
1.396 |
444.27 |
|
02 |
1.329 |
|||
|
03 |
1.264 |
|||
|
Hemosaf |
01 |
1.642 |
1.361 |
432.20 |
|
02 |
1.322 |
|||
|
03 |
1.119 |
Values were calculated as 10 mg of the syrup and are expressed as mean (n=3) and the absorbance was measured 3 times for each of samples.
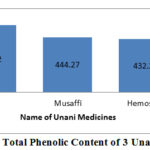 |
Figure 2: Total Phenolic Content of 3 Unani syrups |
Fig. 3 is graphical representing the DPPH free radical scavenging activity of different concentration of 3 unani Samples.
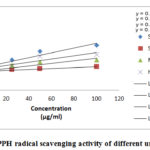 |
Figure 3: DPPH radical scavenging activity of different unani samples |
The concentration has a prominent effect on scavenging activity, in a proportional way concentration increase the DPPH radical activity of 3 samples. For instance, maximum inhibition 85.96 for Hemosaf was spotted at 100 µg/ml and IC50 (µg/ml) was measured 1.91 From the standard curve showed that when the concentration was 100 µg/ml, the inhibition is maximum which is 95.85% and 50% inhibition (radical scavenging) at 1.93 µg/ml.
Table 2: Comparative DPPH radical scavenging activity of 3 Samples and standards of Ascorbic Acid
|
Concentration (µg/ml) |
%Standard and inhibition (in %) of different samples |
|||||||
|
Safi |
Musaffi |
Hemosaf |
Ascorbic acid (Standard) |
|||||
|
12.5 |
39.53 |
IC50 (µg/ml) 99.45 |
36.21 |
IC50 (µg/ml) 33.17 |
45.84 |
IC50 (µg/ml) 1.91 |
47.42 |
IC50 (µg/ml) 1.93 |
|
25.0 |
44.35 |
53.65 |
51.99 |
64.42 |
||||
|
50.0 |
45.34 |
60.13 |
72.42 |
83.03 |
||||
|
100.0 |
49.66 |
64.78 |
85.96 |
95.85 |
||||
Membrane Stabilizing Activity
Different Unani preparation samples subjected to analyze for membrane stabilizing activities were expressed in statistically (Table 2). In hypotonic solution activated conditions, the samples were observed for inhibition of lysis for erythrocyte membrane within the range of 6.86-67.01%. The samples exhibited optimum inhibition of 50.24% hemolysis of RBC respectively as compared to 67.01% showed by acetyl salicylic acid, whereas the minimum inhibition capability was observed at 2.00 mg/ml.
Table 3: Effect of extract of Safi, Musaffi and Hemosaf on hypotonic solution-induced haemolysis of erythrocyte membrane.
|
Treatment |
Concentration (mg/mL) |
Optical density |
% inhibition of haemolysis |
|
Control |
1 |
3.698 |
ˉˉ |
|
Safi |
2 |
3.444 |
6.86 |
|
4 |
2.718 |
26.5 |
|
|
6 |
2.284 |
38.23 |
|
|
8 |
1.840 |
50.24 |
|
|
Musaffi |
2 |
2.839 |
23.22 |
|
4 |
2.413 |
34.74 |
|
|
6 |
2.376 |
35.74 |
|
|
8 |
1.934 |
47.70 |
|
|
Hemosaf |
2 |
3.244 |
12.27 |
|
4 |
2.758 |
25.41 |
|
|
6 |
2.654 |
28.23 |
|
|
8 |
2.409 |
34.85 |
|
|
Acetyl salicylic acid |
0.1 |
1.22 |
67.01 |
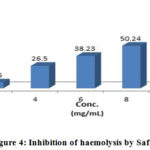 |
Figure 4: Inhibition of haemolysis by Safi |
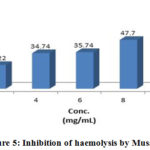 |
Figure 5: Inhibition of haemolysis by Musaffi |
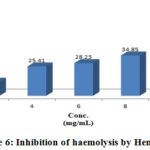 |
Figure 6: Inhibition of haemolysis by Hemosaf |
Discussion
The amount of total phenolic content for Safi, Musaffi and Hemosaf represented in Figure 2 which revealed that the samples have important phenolic property which is further need to research broadly. In this investigation, the samples from unani preparations presented modest antioxidant properties with an IC50 compared to the standard values of a drug commonly used. Antioxidant activity of these different samples was found to increase with the increasing concentration compared to the standard which is also relevant. Lysis of the Red Blood Cell membranes accompanied by the oxidation when accepted to harmful substances such as hypotonic medium through lysis of hemoglobin that cause to release inflammatory agent17. To determine the anti-inflammatory activity of the sample preparations, it is important to consider their potentiality of inhibition16,17. Our current investigation deals with the methanolic samples of the unani preparations which showed potent RBC membrane stabilization activity. So it is easily determined from the gallic acid equivalent in total phenolic content graph that Safi and Hemosaf subsequently contain highest and lowest amount of phenolic content and they reversely exhibit anti-oxidant activity. From Membrane Stabilizing Activity test it can be conclude as, “although Musaffi has large membrane stabilizing capacity than Safi and Hemosaf at low concentration but Hemosaf has the regular stabilizing activity in each concentration”.
Conclusion
In developing countries most of the poor population has to depend on the indigenous systems for the maintenance of their health. This study inspires the increasing use of unani medicines day by day. But the effectiveness of this drugs are not clearly exposed scientifically where most of which are distributed in a raw state and below standard form to the people. The use of such drugs may dangerous and threatens for public health. Thus the analysis of these drugs for exploring its chemical properties and their biological investigation is the present need for standardization of unani medication system. In South Asian less developing country like Bangladesh has to spend a lot of money for importing raw materials and semi or partially processed pharmaceutical drugs of plant origins from other countries and this trend is growing upwards day by day. This huge amount of money would be saved if the use of native medicinal plants or their raw products which are used by the unani and herbal drugs satisfy the public needs and this study is also done for this sense to understand the actual quality of popular unani medicines which is related to public health.
Acknowledgement
The authors are highly acknowledged all the provided facilities; specially laboratory support by the Department of Pharmacy, Noakhali Science and Technology University.
Competing interests
The authors declare that they have no competing interests.
References
- Rahmatullah, M.; Hasan, A.; Parvin, W., Moniruzzaman, M.; Khatun, A.; Khatun, Z.; Jahan, R. African J. Tradit. Complement. Altern. Med. 2012, 9, 350–359.
CrossRef - Yuan, H.; Ma, Q.; Ye, L.; Piao, G. Mol. 2016, 21, 559-576.
CrossRef - Hassan, M.; Ragib, A.; Hossain, M.T; Sazib, S.M.; Islam, M.Z. Nor. Am. Aca. Res. 2019, 3, 1-14.
- , C.T.; George, V.; Ijinu, T.P.; Pushpangadan, P.; Andrae-Marobela, K. Pharmacogn. 2017, 15-30.
- Kaya, H.O. J. African Stud. 2017, 31, Supplement 1.
- Raju, G.S.; Moghal, M.M.R.; Dewan, S.M.R.; Amin, M.N.; Billah, M.M. Avicenna J. Phytomed. 2013, 3, 313-320.
- Ekor, M. Front. Pharmacol. 2014, 4, 177-186.
CrossRef - Astutik S.; Pretzsch J,; Kimengsi, J.N. Sustainability. 2019, 11, 5483-5516
CrossRef - Agbor, G.A.; Vinson, J.A.; Donnelly, P.E. Int. J. Food Sci. Nutr. Diet. 2014, 3, 147-156.
- Blainski, A., Lopes, G.; de Mello, J. Mol. 2013, 18, 6852–6865.
CrossRef - Gupta, M.; Mazumeder, U.K.; Sivahkumar, T.; Vamis, M.; Karki, S.; Sambathkumar, R. Elsevier Inc. 2003, 7, 15-30.
CrossRef - Gupta, M.; Mazumder, U.; Gomathi, P. Asian J. Plant Sci. 2007, 6, 533-537.
CrossRef - Majumder, M.S.; Hossain, D.M.S.; Amin, M.N.; Moghal, M.M.R.; Banik, S.; Hossain, M.M. World J. Pharm. Pharm. Sci. 2014, 3, 58-72.
- Sikder, M.A.; Kaiser, M.A.; Rashid, M.A.; Millat, M.S.; Sultan, A. J. Pharmacol. Phytochem. 2012, 1, 45-50.
- Shinde, U.A.; Phadke, A.S.; Nair, A.M.; Mugantiwar, A.A.; Dikshit, V.J.; Saraf, V.O. Fitoterapia. 1999, 70, 251-257.
CrossRef - Feirrali, M.; Signormi, C.; Ciccolili, L.; Comporti, M. Biochem. J. 1992, 28, 5295-5301.
- Mounnissamy, V.M.; Kavimani, S.; Balu, V.; Quine, S.D. Iranian J. Pharmacol. Therap. 2007, 6, 235-237.

This work is licensed under a Creative Commons Attribution 4.0 International License.









