Kinetic, Thermodynamic and Isotherm Studies for the removal of Acid Red 88 from Aqueous Medium by Polyaniline-CuCl2 Composite
Anusha Dhanagopal, Lingeswari Dheenathayalan and Vimala Thiyagarajan
Department of Chemistry, Seethalakshmi Ramaswami College, Affiliated to Bharathidasan University, Tiruchirappalli-620002, TamilNadu, India.
Corresponding Author Email: vimalsrc@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350621
Article Received on : 30-10-19
Article Accepted on : 07-12-2019
Article Published : 30 Dec 2019
The metal salt doped polyaniline has been used as adsorbent for the removal of synthetic dye. The influence of experimental parameters like association time, adsorbent dosage, agitating speed, pH and electrical conductivity on adsorption of Acid Red 88 was systematically investigated. More than 95% of Acid Red 88 was removed after 55 minutes of adsorbent/adsorbate contact time for 0.3g/L of PANI-CuCl2. Calculations have been carried out to analyse the impact of weight of PANI-CuCl2 composite and concentration of Acid Red 88 on the percentage of decolourisation. Kinetics and isotherm analysis were also carried out to describe the adsorption process. Electrical conductivity, SEM and FTIR technique were used to characterize the adsorbent before and after adsorption. The thermodynamic parameters like changes in free energy, enthalpy and entropy were also evolved to predict the nature of adsorption. The adsorbent is stable, easy to prepare and suitable to remove Acid Red 88 from effluent.
KEYWORDS:Polyaniline; Synthetic Dye; Adsorption; Isotherms; Kinetics and Thermodynamics
Download this article as:| Copy the following to cite this article: Dhanagopal A, Dheenathayalan L, Thiyagarajan V. Kinetic, Thermodynamic and Isotherm Studies for the removal of Acid Red 88 from Aqueous Medium by Polyaniline-CuCl2 Composite. Orient J Chem 2019; 35(6). |
| Copy the following to cite this URL: Dhanagopal A, Dheenathayalan L, Thiyagarajan V. Kinetic, Thermodynamic and Isotherm Studies for the removal of Acid Red 88 from Aqueous Medium by Polyaniline-CuCl2 Composite. Orient J Chem 2019; 35(6). Available from: https://bit.ly/2MuqHfi |
Introduction
Water is a life saving compound which is vital for all living plants and animals. The use of water has increased many folds due to development of technology and population explosion which causes the pollution of water. Chemical industries present nearby water resources pollute rivers, lakes and underground water. Water pollution brings detrimental effect to the human population, aquatic life and plant kingdom. City sewage, effluent from industries like tannery, dyeing units, and metallurgical processes are mixed into the aquatic sources and contaminate the water. Synthetic dyes are chemically and photolytically fast which are extremely persisting in natural environments. Textile industry is a foremost one in staining the water which causes BOD, COD and eutrophication of water. Micro toxicity of water affects the biotic communities.1 Development of treatment methods and continuous search for the best process is ongoing. Adsorption is one of the economically viable and operationally simple methods for the treatment of dye effluent.
Polyaniline exhibits wide varieties of applications in the field of photosensitizers,2 sensor,3 adsorption,4-6 solar cells etc. The present study is an attempt to remove Acid Red 88 (AR 88) from aqueous solution by using PANI-CuCl2 as adsorbent.
Materials and Methods
Materials
Acid Red 88 was procured from EMCO Dye Stuff Pvt. Ltd., Maharashtra. Dye was used as received. Aniline and HCl were obtained from MERCK SPECIALITIES (P) Ltd., Mumbai. Ammonium persulphate was obtained from LOBA CHEMIE PVT.LTD., Mumbai. CuCl2 was obtained from E. MERCK (INDIA) LTD., Mumbai. Structure and details of the dye is shown in the figure-1 and Table-1
Table 1: Properties of AR 88
|
Molecular formula |
C20H13N2NaO4S |
|
Molar mass |
400.38 g/mol |
| CAS number |
23621588 |
| PubChem |
135465069 |
|
ChemSpider |
10483346 |
| λmax |
518 nm |
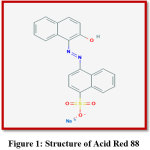 |
Figure 1: Structure of Acid Red 88 Click here to View Figure |
Instrumentation
Batch adsorption studies were carried out in presence of UV light of 6 ampere using RIS-24 BL orbital shaker. The concentration of dye solutions were measured using MAPADA V-1100 D spectrophotometer. Four Probe Setup DFP-03 was used for finding Electrical conductivity. Equip-Tronics pH meter was used to measure pH.
Methods
Preparation of PANI-CuCl2
Copperchloride doped polyaniline was synthesized by chemical oxidation coupled with polymerization, where ammonium persulphate was used as an oxidant.7 10g of ammonium persulphate dissolved in 20ml distilled water was added into the solution of aniline(5ml) dissolved in 1.5M HCl (70ml). To that aliquote 5ml of 8% CuCl2 was added. The solution mixture was stirred at 400rpm for 5 hours at room temperature. After the polymerization PANI-CuCl2 granules was obtained.
Preparation and Standardization of Dye Solution
Stock solution of Acid red 88 was prepared in distilled water (1g/L). Acid red 88 was standardized by measuring the optical densities of various concentrations of the dye solution at 518nm using visible spectrophotometer.
Adsorption studies
The effect of significant parameters on the rate of adsorption like contact time, concentration of the dye solution (9 to 21mg/L), pH (4–9), adsorbent dosage (0.1 to 0.7 g/L), agitation speed (100-250) and temperature (303–323 K) were analysed for the adsorption of AR 88. Appropriate quanitity of PANI-CuCl2 composite and acid red 88 are mixed to 100ml in water in a 250ml beaker. The mixture was agitated at 250rpm in orbital shaker at 30°C. Concentration of unadsorbed dye was measured using UV Vis spectrophotometer at 518nm. Equilibrium quantity of AR 88 was measured using the formula.
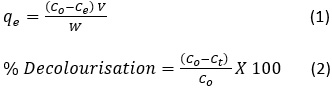
Co the dye initial concentration
Ct (mg/L) concentration of AR 88 at different time interval t(min.)
qe (mg/g)- quantity of AR88 adsorbed dye amount per unit mass of the adsorbent at equilibrium,
W(g) is the amount of adsorbent used.
Desorption
Results and Discussion
The influence of contact time on the batch adsorption of dye was carried out at 30°C. Removal of AR 88 on PANI composite was rapid in the initial stage, the quantity of dye adsorbed gradually increased, afterwards and then no change in amount adsorbed which indicate that the equilibrium reached8 is shown in figure -2. The successive increase in adsorption and attainment of equilibrium state was obtained at 55 min for (AR 88), because of the limited mass transfer of dyes from the bulk solution to the external surface of the adsorbent.
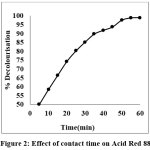 |
Figure 2: Effect of contact time on Acid Red 88 Click here to View Figure |
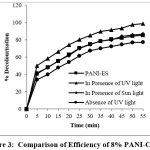 |
Figure 3: Comparison of Efficiency of 8% PANI-CuCl2 Click here to View Figure |
Efficiency of 8% PANI-CuCl2 composite and PANI-ES compared under similar condition for the removal AR 88 which reveal that CuCl2 doped PANI has higher efficiency than PANI-ES. The adsorption study is carried out in the presence and absence of UV light illustrated that UV light assisted the rate of decolourisation under similar condition. The adsorption study is carried out by exposing the reaction aliquot to sunlight alone, remove the dye at a slower rate but complete removal is possible is shown in figure-3. AR 88 is not decolourised under any of the above conditions without addition of adsorbent.
The effect of variation in initial dye concentration on the removal of AR 88 by PANI- CuCl2 was investigated at different concentration ranges from 9 to 21 mg/L (AR88). The percentage removal of dye is increased with decrease in initial dye concentration whereas the qe increases with increase in initial dye concentration and the time required to reach equilibrium is also increased9,10 is graphically shown in the figure 4. At higher initial concentration of dye there may be a strong interaction between PANI-CuCl2 composite and dye molecules. But above optimal concentration of dye the active sites required for adsorption is less which may cause the reduction in rate of decolourisation.
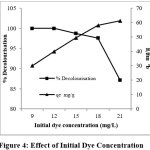 |
Figure 4: Effect of Initial Dye Concentration Click here to View Figure |
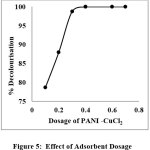 |
Figure 5: Effect of Adsorbent Dosage Click here to View Figure |
The amount of PANI-CuCl2 used in colour removal process is a vital criterion because it decides the holding power of adsorbent for given dye concentration. The impact of adsorbent dosage was evaluated by changing the amount of PANI- CuCl2 from 0.1g/L to 0.7g/L for Acid Red 88 dye at a concentration of 15mg/L. Increasing the adsorbent dosage, augment the contact surface of adsorbent particle and enhances the number of sites available for adsorption11 (Figure 5). Thus, the dose of adsorbent is increased; the higher is the access for adsorbate and increase the chance for adsorption which can be inferred from figure 5.
The effect of variation of variation of temperature on adsorption of dyes on CuCl2 doped PANI composites was studied at 30, 35, 40 and 45°C by keeping dosage of adsorbent, dye concentration, pH and stirring rate constant. It was indicated that the adsorption equilibrium of all dyes decreased with the increase in temperature (figure 6) indicating the exothermic behaviour of adsorption12,13. This may be due to an increase in movement of dye molecule, greater aqueous solubility of dyes which reduces the adsorption process and increase in temperature may decrease the electrostatic interaction between the dye molecule and surface of the adsorbent.
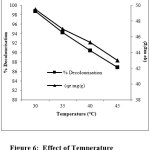 |
Figure 6: Effect of Temperature Click here to View Figure |
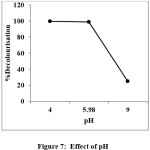 |
Figure 7: Effect of pH Click here to View Figure |
The quantity of dye adsorbed was evaluated as a function of pH in the range 4 to 9. The pH of the dye solution was maintained over the range using 0.1 N HCl or 0.1 N NaOH solution after the addition of adsorbent. Adsorbent dosage, concentration, temperature, stirring rate and volume of dye solution are kept constant to analyse the effect of pH. The plot of percentage removal versus pH is the figure 7.Effect of pH provides possible information about the sorbate and sorbent interaction and the dissociated dye anions at acidic pH attracted by the positively charged adsorbent whereas at alkaline pH adsorption decreased because the negatively charged PANI EB may repulse the dye molecules.14
Increase in round per minute increases the rate of decolourisation and it is approximately twice when the speed increases from 100 RPM to 250 RPM which may be due to reduction of resistance at the boundary of the adsorption of dye molecules by the adsorbent.15,16 This can be seen in the figure 8
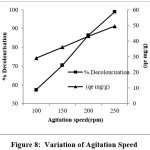 |
Figure 8: Variation of Agitation Speed Click here to View Figure |
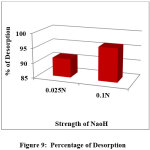 |
Figure 9: Percentage of Desorption Click here to View Figure |
The desorption trend of AR 88 at different strength of NaOH was opposite to that of adsorption process. An increase in the strength of NaOH solution from 0.025 to 0.1N increases desorption efficiency from 91 to 96 %. AR 88 is anionic in nature, adsorption of dye in acidic medium and regeneration of colour in alkaline medium showed that AR 88 was held by the adsorbent, most probably by ion exchange. Reusability study revealed that the dye is desorbed in NaOH and reusability is observed and the percentage of dye decolourised is gradually reduced in successive cycles. This also proves the efficiency of PANI-CuCl2 composite as an effective adsorbent. Figure 9 and 10 shows the percentage of desorption and percentage of readsorption graphically.
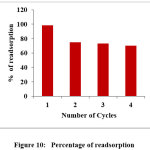 |
Figure 10: Percentage of readsorption Click here to View Figure |
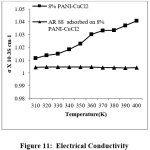 |
Figure 11: Electrical Conductivity Click here to View Figure |
The electrical conductivity of pristine and dye loaded PANI-CuCl2 composite were measured separately and it is found that electrical conductivity of polyaniline-CuCl2 composite is higher than dye adsorbed composites. This imply that the dye accumulated on the superficial area of the polymer composite gives rise to a lean sheet of low doped material because of the removal of the counter ions which leads to drop in electrical conductivity (Figure 11)
Isotherm Studies
The relationship between the amount of a substance adsorbed at constant temperature and its concentration in the equilibrium solution is called the adsorption isotherm.
The linearized form of Langmuir equation is
![]()
Where Q0 & b are Langmuir constants related to maximum monolayer coverage capacity and b is the constant related to the free energy of adsorption17.RL value calculated from the Langmuir equation predict the adsorption is favourable ( 0 <RL<1).
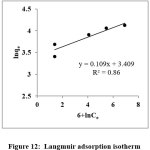 |
Figure 12: Langmuir adsorption isotherm Click here to View Figure |
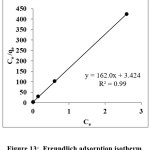 |
Figure 13: Freundlich adsorption isotherm Click here to View Figure |
The mathematical expression relates the equilibrium of dye solution and heterogeneous surface of the PANI-CuCl2 is described by Freundlich isotherm.
The equation can be expressed in its linear form as:
![]()
When lnqe is plotted against lnCe a straight line with a slope 1/n and intercept lnKF as obtained is shown in figure 13. The values of Freundlich constant, n is 9.17 (1/n is lower than 1) reveals that the surface of the adsorbent is heterogeneous and adsorption occurred spontaneously. The analysed adsorption is favorable adsorption18.
Table 2: Isotherm models and their calculated constant values
|
Isotherms |
Linear form of equation | Plot | R2 Value | Calculated values |
| Freundlich | lnqe=lnKf+1/nlnCe | lnCe vs lnqe | 0.860 |
n= 9.17 ; KF = 30.23 (L/g) |
|
Langmuir |
Ce/qe= (1+b Ce)/Q0b | Ce vs Ce/qe | 0.999 | Q0 = 6.17mg/g; b= 47.17(L/mg); RL = 0.001 |
| Temkin | qe = BTlnKT + BTlnCe | lnCe vs qe | 0.910 |
BT = 504.84KJ/mol; KT=298.87L/mg |
Temkin Isotherm
Equation (5) represents the Temkin isotherm19 and the calculated BT indicates that there is a strong interaction between AR 88 and PANI-CuCl2.
qe = BTlnAT+ BTlnCe (5)
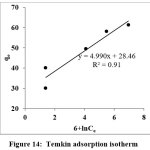 |
Figure 14: Temkin adsorption isotherm Click here to View Figure |
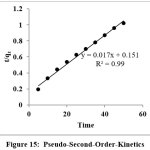 |
Figure 15: Pseudo-Second-Order-Kinetics Click here to View Figure |
Adsorption Kinetics
Kinetic aspects of adsorption provide useful data for determining the feasibility of scale-up operations and reliable fact on the mechanism of sorption. The linear form of pseudo second order kinetic equation is,
![]()
The plot of t/qt versus T gave the perfect straight line which is shown in the Figure 15. From the plot of t/qt versus T, K2 and qe are determined. The correlation coefficient (R2) value is high which shows greater accuracy and this process follows Pseudo-second-order kinetics (Table-4).
Table 3: Kinetic Data
|
Dye |
qe(mg/g) Experimental | qe(mg/g) Calculated | K2 | R2 |
| RB 8 | 49.5 | 58.82 | 1.91×10-3 |
0.999 |
Intra Particle Diffusion
The equation for intraparticle diffusion is expressed as follows,
qt= Kid(t)0.5+C (7)
Where qt (mg/g) is the amount of dye ions adsorbed onto 8%PANI- CuCl2 at t and Kid (mg/ (g.min)) is the intra particle diffusion rate constant. As shown in the figure the plot exhibited a break, initial sharp line corresponds to the adsorption of dye to the external surface of PANI-CuCl2 and the second part of the plot related to the gradual adsorption stage of the interior portion of the adsorbent.
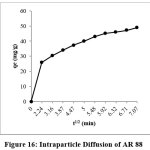 |
Figure 16: Intraparticle Diffusion of AR 88 Click here to View Figure |
Thermodynamic parameters
Thermodynamic parameters like changes in Gibbs free energy ( KJmol-1), enthalpy ( KJmol-1) and entropy ( KJmol-1) were calculated using the following equation
![]()
Where Kd is the equilibrium constant, the values of ∆H° and ∆S° were calculated from the slope and intercept of Van’t Hoff plots of ln Kd versus 1/T. The ∆H° value for AR 88 calculated from Van’t Hoff equation is -115 KJ mol-1. The negative value of ∆H° indicates that the overall adsorption process was exothermic in nature. The negative value of ∆S°(-335 KJ mol-1) shows that there is decrease in disorder of the system, thus we can tell that electrostatic binding between adsorabte and adsorbent in adsorption process.
The value of Gibb’s free energy was calculated at four different temperatures according to the equation
DG°=-RT ln KL (9)
The negative value of ∆G° indicates the spontaneous nature of the adsorption process. The calculated values are given in the Table 5.
Activation energy is calculated from the Arrhenius equation
ln k = lnA− Ea/RT (10)
(Ea) is calculated from the slope of plots of ln k versus 1/T is found to be 109KJ mol-1 which also supports adsorption21.
Table 4: Calculated thermodynamic parameters and their outcome results
|
Thermodynamic parameters |
Calculated values for AR 88 | Outcome results |
| ∆G° | -6.751 KJ mol-1 -6.863 KJ mol-1 -6.974 KJ mol-1 -7.086 KJ mol-1 |
Spontaneous and feasible in nature |
|
∆S° |
-335 KJ mol-1 | Associative mechanism |
| ∆H° | -115 KJ mol-1 |
Exothermic in nature |
FTIR Analysis
FTIR spectral analysis of the unloaded 8% PANI-CuCl2 composite shows a characteristic peak at 3434 cm-1 due to the N-H stretching vibration which is absent in AR 88 loaded PANI-CuCl2 composite. The dye treated polymer composite showed some new peaks and few peaks shifted which can be inferred from figure ( 17(a) and 17(b)).
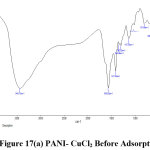 |
Figure 17(a): PANI- CuCl2 Before Adsorption Click here to View Figure |
 |
Figure 17(b): PANI- CuCl2 After Adsorption of AR 88 Click here to View Figure |
Scanning Electron Microscopy
Recorded SEM images of PANI-CuCl2 composite before and after adsorption of AR 88 are porous and irregular and smooth respectively (figure 18(a) & 18(b)) which also support that the adsorbent is loaded with dye.
 |
Figure 18(a): PANI- CuCl2 Before Adsorption Click here to View Figure |
 |
Figure 18(b): PANI- CuCl2 After Adsorption of AR 88 Click here to View Figure |
Conclusion
All the evidences from the analysed result showed PANI-CuCl2 is a viable adsorbent to remove Acid Red 88 from the effluent.
Acknowledgement
The authors would like to thankthe management of Seethalakshmi Ramaswami college, Tiruchirappalli, TamilNadu-620002 for the encouragement and the opportunity to carry out this work.
Conflict of Interest
The authors declared that there is no conflict of interest regarding the publication of this paper.
Refrences
- Sekhar, C.P.; Kalidhasan, S.; Rajesh, V.; Rajesh, N. Chemosphere, 2009, 77, 842-847.
- Mitra, M.; Ghosh, A.; Mondal, A.; Kargupta, K.; Ganguly, S.; Banerjee, D., Appl. Surf. Sci., 2017 402 418.
- Baker, C.O.; Huang, X.; Nelson, W.; Kaner, R.B. Chem. Soc. Rev., 2017 46 1510-1525.
- Ayad, M.; El-Hefnawy, G.; Zaghlol, S. Chem. Eng. J. 217 (2013) 460–465.
- Ayad, M.M.; El-Nasr. A.A. J. Phys. Chem. C 114 (2010) 14377–14383.
- Ayad, M.M.; Abu El-Nasr, A.; Stejskal, J. J. Ind. Eng. Chem. 18 (2012) 1964–1969.
- Bingol D.; Sevil V.; Sibel Z.; & Utkan O.; Synthetic metals, 2012 162 1566-1577.
- Aghayan.H; Mahjoub A.R;Khanchi A.R; Application surface science, 2012 261 14-20
- Pham T.D; Kobayashi.M; Adachi.Y, Polymer Science, 2015 293 1877-1886.
- Yao .Y;Xu.F;Chen.M;Xu.Z;Zhu.Z; Bioresource Technology 2010 101 3040-6
- Ansari.R; Mohammad Khah .A; Mosayebzadeh .Z, Annals of Biological research , 2011 2 323-328
- Wang, L.; Zhang, J.; Wang A., Desalination 2011 266 33–39
- Sarkar, K.; Banerjee, S.L.; Hydrol. Curr. Res.2012 3 133.
- Mahanta.D.; Madras.G.; Radhakrishnan.S.; Patil.S.; .Phys.Chem.B., 2009 113 2293-2299.
- Bharathi K; Ramesh S; Appl Water Sci 2013 3 773–790.
- Weng CH; Lin YT; Tzeng TW; J Hazard Mater 2009 170, 417–424.
- Joo JB; Park J; Yi J; J Hazard Mater 2009 168, 102–107.
- Monash P; Pugazhenthi G ; Adsorption 2009 15 390–405.
- Mahmoodi NM; Khorramfar S; Najafi F; Desalination 2011 279, 61–68.
- Erol Alver; Aysegül Metin ; Chem. Eng. J.,2012 200–202 59–67
- BrajaN.Patra; Muralidhar Patra; Deola Majhi; Nilaya Kumar Mohanty; Polymer science,2015 57(4), 349-354

This work is licensed under a Creative Commons Attribution 4.0 International License.









