Green Synthesis of Silver Nanoparticles by Sonicated Method Using Pulicha Indeca Stem Extract: Characterization and Study of Antibacterial Activity, Anticancer Activity and Cell Viability Test
Gugulothu Yaku, Venkateswerlu and TVD Prasad Rao
Department of Chemistry, Osmania University, Hyderabad - 500 007, TS, INDIA
Corresponding Author Email: thyp123@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350620
Article Received on : 21-11-19
Article Accepted on : 04-12-2019
Article Published : 23 Dec 2019
In the present work, green synthesized silver nanoparticles by sonofication method using pulicha extract, which can act as reducing as well as stabilizing agent. The silver nanoparticles were characterized by UV-VIS spectra, FTIR, XRD, SEM and TEM analysis. The UV –VIS SPR peak was observed at 440 nm, which represents the characteristic plasmon resonance of nanostructures. The fcc crystalline structure of silver nanoparticles is evident from XRD studies. The shape and size of synthesized AgNPs were studied by TEM. The synthesized AgNPs, were mostly mono-dispersed and spherical in shape. The particles are well separated from each other and did not exhibit any aggregation. This indicates the effective capping nature of pulchea indeca extract. The AgNPs size distribution histogram and the average size of AgNPs was found to be 14 ± 2 nm. The effect of silver ion concentration range from 1mm to 10mm and extract concentration 1% to 8% has been studied on the formation of silver nano particles. The AgNPs shows positive antibacterial acivity against stayphylococusaureoous , klebisellapneumonia, basilusbtilis and negative antibacterial activity against protious mirabilis bacteria studied. The anticancer activity of AgNps shows positive activity on HeLa cell.
KEYWORDS: Download this article as:| Copy the following to cite this article: Yaku G, Venkateswerlu, Rao TVD P. Green Synthesis of Silver Nanoparticles by Sonicated Method Using Pulicha Indeca Stem Extract: Characterization and Study of Antibacterial Activity, Anticancer Activity and Cell Viability Test. Orient J Chem 2019; 35(6). |
| Copy the following to cite this URL: Yaku G, Venkateswerlu, Rao TVD P. Green Synthesis of Silver Nanoparticles by Sonicated Method Using Pulicha Indeca Stem Extract: Characterization and Study of Antibacterial Activity, Anticancer Activity and Cell Viability Test. Orient J Chem 2019; 35(6). Available from: https://bit.ly/396zdL6 |
Introduction
Silver nanoparticles (AgNPs) are widely applicable in pharmaceuticals, health care industrial and consumer care etc. due to their specific physical and chemical features(1-3), Owing to specific features of silver nanoparticles, they are used as antibacterial agents, s and anti-cancer (agents) drugs(4). Silver nanoparticles have been used in textiles, biomedical, domestic (2,5,6). Silver nanoparticles are unique in nature due to its surface to volume ratio, hence they can be used in different applications (7,8).
Nanoparticles are important in pharmaceutical industries and bio technological industries. Silver nanoparticles exhibits antibacterial, anticancer, antifungal, anti plasmodial and anti larvacidal properties [9-13]. Silver nanoparticles are less toxic to animal cells.and most efficient anti bacterial activity.
For the synthesis of AgNps different methods (14,16-18) have been adopted. The conventional physical and chemical methods are more cost effective and harmfull.Generally, chemical stabilizers (18-22) can be used for in the synthesis of AgNps. However, some of chemical stabilizers are toxic and able to pollute the environment
The green chemistry exhibits much interest for the synthesis of silver nanoparticles. In different biological synthesis methods, of the AgNps, are having definite size and morphology.Sincere efforts are made to synthesise nanoparticles using natural products as stabilisers (14,15,17) and plant extracts [23,24] as stabilizing agent. Hydroxy groups in natural products can act as reducing as well as stabilisiing agents.
Cancer is multi factoial desease, that is treated by various treatments including chemotherapy, surgery, radiation, and targeted therapy [25]. So the challenge is cost effective, cell targeted AgNps can be synthesized for the therapeutic usage as antcancer agent drugs.
In the present work synthesized AgNps, using pulichea indeca extract which can act as reducing as well as stabilizing agent.The AgNps are characterized by using different techniques. The anti bacterial activity and anti cancer activity for the AgNps have been studied. Finally these studies gives future perspective for the AgNps.
Materials and Methods
Fresh and healthy of pulchea indica stems were collected from the local area near mancheryala, adilabad (dist), TS India. A.R Grade (99.9%) of Silver Nitrate (Merck) and The Cancer cell lines from NCCS, Pune, were purchased In this study DD H2O is used as solvent for further sample analysis.
Methods
Preparation of pulchea indeca extract
About 10gms of pulchea indeca stem cleaned with tap water followed by DD H2O and then stems were collected. The washed stems are thoroughly dried under sun light to remove hygroscopic (unwanted material) nature from the stems. Further the dried stems were chopped into small species and grinded to get the fine powder. Nearly 10gms of fine powder is added to 250 ml of DD H2O and heated by using magnetic stirrer. After continuous stirring for 30 min at 1200C, the extract was filtered at 6000 rpm. The extract is stored at 50C for further usage.
Synthesis of AgNPs
For the Synthesis of AgNPs, different samples were prepared by mixing 20ml of AgNO3 solutions having concentration from 0.1mM, to 1.0mM each with 60 ml of pulchea indeca extract. And 60ml of l%, 2%, 3%, 4%, 5% 6%, 7% and 8% stem extract solutions are mixed each with 20ml of AgNO3 solution.
Characterization
After color change of AgNps solutions, the synthesized AgNps were analyzed by measuring the Surface Plasmon Resonance (SPR) at a wavelength range between 200 and 800 nm by the UV Spectroscopy FT-IR spectra for Stem extract alone and stem capped AgNPs were recorded separately by using an FT-IR spectrophotometer. Powder X-ray Diffraction (XRD) measurements AgNPs were carried out in X’pert Pro-powder X-ray diffractometer. The morphology and size distribution measurements of the AgNPs were carried out by (HR-TEM, FEI Company, USA).
Antibacterial Activity Test
The synthesized AgNps solution were taken and made into aliquots of 25µl, 50µl, 75µl, 100µl by dissolving them in DMSO (solvent) for MIC assay in case of powdered samples and for liquid samples used directly.
Antibacterial Assay
The antibacterial assay was carried out by performing pour plate method in which 1ml bacterial active cultures per plate were mixed into agar media before solidifying temperature and poured into plates. Wells were made by using sterile well borer and samples loaded 25µl 50µl 75µl 100µl µl of each respectively, in Gram positive and Gram negative plates. Plates were incubated at 37oC degrees for 36 hours. In antibacterial study, four Gram positive and Gram negative bacteria (a) Staphylococcus aureous (Gram-positive), (b) Klebsiella eumoniae, (Gram-negative) (c) Bacillus subtitles (Gram-positive), and (d) Proteus mirabilis (Gram-negative) are used.
Results and Discussion
UV-Vis spectroscopy
The UV-Vis spectrum of synthesized AgNPs showed the absorption peak at 440 nm indicated the formation of Nano metallic silver particles. Pulchea indeca stem extract contains flavonoids, alkaloids and proteins could reduce silver ions in the reaction solution and stabilizes the synthesized AgNPs. The formation of pulchea indeca -AgNPs was confirmed by a colour change from colorless to yellow color. A sharp and a narrow distinct peak are absorbed at λmax = 440 nm in the ultra-visible region, clearly elucidates the formation of AgNPs within a short duration of time (10–15 min). The role of pulchea indeca concentration and AgNO3 concentrations, were studied over the synthesis of AgNps. Fig 1a shows the effect of AgNO3 concentrations (0.1mm to 1.0mMon the synthesis AgNps. The effect has been studied by sonication method using different concentrations of AgNO3 (0.1- 0.10%) mixed with 5 % of plant extract of pulchea indeca for 15 min of time (1:3ratio). And it indicates the formation of AgNPs increases with increasing the concentration of AgNO3. This may be due to increase in the number of silver ions with the increase in concentration of AgNO3. Absorption spectra of AgNPs prepared at different pulchea indeca concentration are presented in Fig.1b. It reveals that the AgNPs increases with the increases concentration of the pulchea indeca. The synthesis was also evaluated by mixing different concentration of pulchea indeca (1% to 8%) with 0.5mM concentration of AgNO3 (3:1ratio) for 15 min time (Fig.1b). With the increase in the concentration of pulchea indeca plant extract from 1% to 8%, the SPR absorption band intensities are increased. This may be due to availability number of flavonoids, alkaloids and proteins in the extract are reducing more number of silver ions into more number of silver nanoparticles.
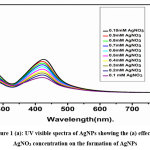 |
Figure 1 (a): UV visible spectra of AgNPs showing the (a) effect of AgNO3 concentration on the formation of AgNPs Click here to View Figure |
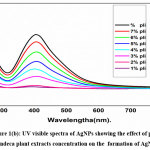 |
Figure 1(b): UV visible spectra of AgNPs showing the effect effect of pulchea indeca plant extracts concentration on the formation of AgNPs Click here to View Figure |
FTIR Analysis of AgNPs
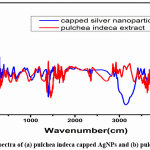 |
Figure 2: FTIR spectra of (a) pulchea indeca capped AgNPs and (b) pulchea indeca alone. Click here to View Figure |
FTIR studies was carried out to analyse the pulchea indeca extract alone and the capping/stabilized AgNPs which are formed by the reduction of silver ions of AgNO3 with the flavonoids, alkaloids and proteins present in the pulchea indeca extract The FTIR spectra of pulchea indeca extract alone (Fig.2 b) exhibited stretching vibrations at 3300, 2940, 1750, 1360 and 1011 cm-1, while the pulchea indeca extract capped/stabilized AgNPs (Fig.2 a) shows the characteristic stretching frequencies at 3340, 2953, 1745, 1610, 1360 and 1020 cm-1. The broad peak at around 3300 cm-1 in figure. a corresponds to the -OH stretching vibrations of flavonoids. The peak at around 2940 cm-1 belongs to -CH stretching, and strong peak at around 1750cm-1 can be assigned to the carbonyl stretching. Further the peaks at around 1011 cm-1 can elucidate to the C–O stretching. FTIR spectra of pulchea indeca extract capped/stabilized AgNPs shows some clear individual from that of FTIR spectra of pulchea indeca extract alone. Most importantly, the intensity of -OH stretching vibration is reduced and the intensity of carbonyl stretching got increased.
XRD analysis of AgNPs
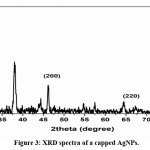 |
Figure 3: XRD spectra of a capped AgNPs. Click here to View Figure |
The XRD pattern of AgNPs (fig.3) using pulchea indeca extract and peelings extract capped AgNPs were carried out by coating the colloidal stable samples under the reduced pressure. In the XRD spectrum, the Bragg peaks at 2θ = 33.2°, 48.5°, 65.6° and 78.8° for 111, 200, 220, 311 planes were identified the crystalinity of pulchea indeca extract capped AgNPs (Fig.3). These values agreed with reported fcc crystalline silver nanoparticles. The X-ray diffraction studies indicate the presence of phytochemicals in the pulchea indeca extract solution.
Surface Morphological Study (FE-SEM).
 |
Figure 4: a&b FE-SEM images of AgNPs. Click here to View Figure |
Morphology of AgNPs were studied from FE-SEM images
From the FE-SEM images of the Ag nanoparticle are nearly spherical shape. There were a few Ag nanoparticles having oval shape as well. According to these studies, maximum number of the nanoparticles is having an average diameter and size is less than 50 nm. It indicates that maximum nanoparticles are uniform in nature .and these Nano-particles were formed by Sonicated assisted green method. The size distribution of 25-40nm and an average diameter about 14± 2 nm of silver nanoparticles, indicates that AgNPs were bio-synthesized by using dried pulchea indeca extract. According to EDX spectrometers analysis the presence of the elemental Ag signals, corresponding to the Ag nanoparticles were confirmed.
Surface Morphological Study (EDX).
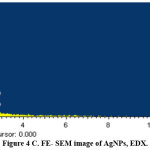 |
Figure 4: C. FE- SEM image of AgNPs, EDX. Click here to View Figure |
TEM images of AgNps and particle size distribution.
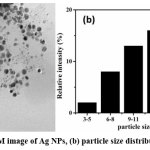 |
Figure 5: a TEM image of Ag NPs, (b) particle size distribution of AgNps. Click here to View Figure |
The shape and size of synthesised AgNPs were studied by TEM. The fig 5a shows that synthesized AgNPs, were mostly mono-dispersed and spherical in shape. The particles are well separated from each other and did not exhibit any aggregation. This indicates the effective capping nature of pulchea indeca extract. The histogram AgNPs size distribution of (fig 5b) was constructed and the average size of AgNPs was found to be 14 ± 2 nm.
Antibacterial activity of silver nanoparticles.
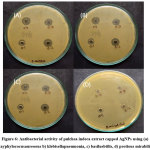 |
Figure 6: Antibacterial activity of pulchea indeca extract capped AgNPs using (a) stayphylococusaureoous b) klebisellapneumonia, c) basilusbtilis, d) protious mirabilis. Click here to View Figure |
The antibacterial activity of AgNPs was studied against a) stayphylococusaureoous (gram negative), b) Bacillussubtilis (gram positive), c) Klebsiella pneumoniae (gram negative) and d) protious mirabilis (gram negative) by agar well diffusion method (Figure.6). The zone of inhibition of four bacteria for AgNps are given in table (1). the AgNPs are directly binded with DNA structure of gram negative bacteria and it ruptures and deform the structure and thus, kills bacteria. Green Synthesized AgNPs shows better antibacterial activity for Silver Nanoparticles, which makes contact with a greater amount of bacteria and destruct the bacteria. As calculated from structural study, better antibacterial activity is obtained at 100 mL of prepared AgNPs against all four bacteria studied. Thus, all the factors shows good antibacterial activity for synthesized AgNPs.
Table 1. Zone of inhibition (in cm) for the bacteria at different concentration of the AgNps
|
S.no |
Name of bacteria | 25µl | 50µl | 75µl | 100µl |
| 1 | Stayphylococus aureoous | 0.2 | 0.3 | 0.3 |
0.4 |
|
2 |
Bacillussubtilis | 0.3 | 0.4 | 0.4 | 0.6 |
| 3 | Klebsiellapneumoniae | 0.3 | 0.6 | 0.6 |
0.8 |
|
4 |
protious mirabilis | – | – | – |
– |
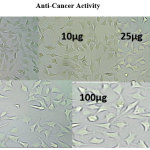 |
Anti-cancer activity Click here to View Image |
The anti cancer activity of test compounds are determined by using MTT assay.
Table 2: Absorbance, percentage of inhibition, % of viability and IC50 values at different concentration of silver nanoparticles in the study of anticancer activity
|
Concentration (µg) |
Absorbance at 570nm | % Inhibition | % Viability | IC50 (µg) |
| 5 | 0.552 | 24.59 | 75.41 |
49.34 |
|
10 |
0.421 | 42.48 | 57.52 | |
| 25 | 0.352 | 51.91 |
48.09 |
|
|
50 |
0.302 | 58.74 | 41.26 | |
| 100 | 0.317 | 57.37 |
42.63 |
|
|
Untreated |
0.732 | 0 | 100 | |
| Blank | 0 | 0 |
0 |
Measurement of cell viability and proliferation forms the basis for numerous in vitro assays of a cell population’s response to external factors. The MTT Cell Proliferation Assay measures the cell proliferation rate and conversely, when metabolic events lead to apoptosis or necrosis, the reduction in cell viability.
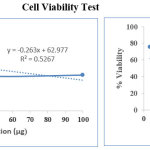 |
Cell viability test Click here to View Image |
Table 3: Test compounds treated with HeLa cells showing the IC 50values are as shown in the table
|
Sr. No |
SAMPLE NAME | IC50 (µg) |
|
HeLa |
||
| 1 | AgNPs 100 µg |
49.34 |
The AgNps has anti cancer activity (49.34) against Hela cell at 100 µg concentration of silver nanoparticles
Conclusions
Then synthesis of stable AgNPs was achieved without adding any external reagents. It is Efficient and eco-friendly renewable ‘‘green’’ approach has been established for the green synthesis of AgNPs. The synthesis is carried out using DD H2O, pulchea indeca plant extract with sonicated method. The pulchea indeca plant extract used as a reducing / stabilizing agent without using any harmful chemicals and synthetic reagents. The concentration of pulchea indeca plant extract and concentration of Ag NO3 solutiion affected the amount of formation of AgNPs. The antibacterial activity studies of AgNPs reveals that the synthesized AgNPs shows positive antibacterial activity against stayphylococusaureoous (gram negative), Bacillussubtilis (gram positive) and Klebsiella pneumoniae (gram negative) bacteria. Synthesised AgNPs shows nagative antibacterial activity against protious mirabilis (gram negative) bacteria.the silver nanoparticles exibits positive anticancer activity against Hela cancer cells (49.34). Such cheap source of material gives an opportunity to a cost-effective and ecofriendly preparation of stable AgNPs having various potential applications to be used.
Acknowledgements
Y.G. gratefully acknowledges RAJIV GANDHI NATIONAL FELLOWSHIP. The authors acknowledge DST-FIST, and the authors are thanks full to MRC, MNIT Jaipur, STICK cochin kerala. for extending various analytical facilities. And my sincere thanks to Dr.Nagamani. P, Dept of microbiology, Osmania University for providing an antibacterial activity. And my heart full thanks to Dr.Rajkumar.Bandi, postdoc fellow at South Korea, forest science dept. Authors are Sincere thanks full to Department of Chemistry, and CFRD-OU.
Conflict of Interest
The authors declare no conflict of interest in this writing
Rreferences
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. J. Nanomed. 2015, 10, 4203–4222
- Li,W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y.B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Microbiol. Biotechnol. 2010, 8, 1115–1122.
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Renu, P.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001, 1, 515–519.
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Int. Ed. 2013, 52, 1636–1653.
- Li, C.Y.; Zhang, Y.J.; Wang, M.; Zhang, Y.; Chen, G.; Li, L.; Wu, D.; Wang, Q. In vivo real-time visualization of tissue blood flow and angiogenesis using Ag2S quantum dots in the NIR-II window. Biomaterials 2014, 35, 393–400.
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. Colloid Interface Sci. 2004, 275, 177–182.
- Li, L.; Hu, J.; Yang, W.; Alivisatos, A.P. Band gap variation of size- and shape-controlled colloidal CdSe quantum rods. Nano Lett. 2001, 1, 349–351.
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface ,2009, 145, 83–96.
- Gurunathan S., Park J.H., Han J.W., Kim J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. J. Nanomed. 2015;10:4203–4222. doi: 10.2147/IJN.S83953.
- Li W.R., Xie X.B., Shi Q.S., Zeng H.Y., Ou-Yang Y.S., Chen Y.B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010;8:1115–1122. doi: 10.1007/s00253-009-2159-5.
- Mukherjee P., Ahmad A., Mandal D., Senapati S., Sainkar S.R., Khan M.I., Renu P., Ajaykumar P.V., Alam M., Kumar R., et al. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001;1:515–519. doi: 10.1021/nl0155274.
- Chernousova S., Epple M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013;52:1636–1653. doi: 10.1002/anie.201205923.
- Li C.Y., Zhang Y.J., Wang M., Zhang Y., Chen G., Li L., Wu D., Wang Q. In vivo real-time visualization of tissue blood flow and angiogenesis using Ag2S quantum dots in the NIR-II window. Biomaterials. 2014;35:393–400. doi: 10.1016/j.biomaterials.2013.10.010.
- Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: A case study on coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012
- Li, Y. Wang, Q. Yu, Significant parameters in the optimization of synthesis of silver nanoparticles by chemical reduction method, J. Mater. Eng. Perform.,2010, 19, 252–256.
- Kheybari, S.V. Hosseini, A. Fazeli, M.R. Fazeli, Synthesis and antimicrobial effects of silver nanoparticles produced by chemical reduction method, DARU 18 ,2010, 168–172.
- Song, S. Lee, T. Park, B. Lee, Preparation of colloidal silver nanoparticles by chemical reduction method, Korean J. Chem. Eng. 2009,26, 153–155.
- Kempa, R. Farrer, M. Giersig, J. Fourkas, Photochemical synthesis and multiphoton luminescence of monodisperse silver nanocrystals, Plasmonics 1 2006 45–51.
- K. Sahoo, S.S. Kalyan Kamal, T.J. Kumar, B. Sreedhar, A.K. Singh, S.K. Srivastava, Synthesis of silver nanoparticles using facile wet chemical route, Def. Sci. J. 200959 (4).
- -K. Liu, X.-H. Yang, X.-G. Tian, Preparation of silver/hydroxyapatite nanocomposite spheres, Powder Technol. ,2008, 184, 21–24.
- Song, S. Lee, T. Park, B. Lee, Preparation of colloidal silver nanoparticles by chemical reduction method, Korean J. Chem. Eng. 2009, 26 ,, 153–155.
- Kan, C. Wang, J. Zhu, H. Li, Formation of gold and silver nanostructures within polyvinylpyrollidone (PVP) gel, J. Solid State Chem. 2010, 183, 858–865.
- Carotenuto, Synthesis and characterization of poly(N-vinylpyrrolidone) filled by monodispersed silver clusters with controlled size, Appl. Organomet. Chem., 2001, 15, 344–351.
- D. Rivera-Rangel, M.P. González-Muñoz, M. Avila-Rodriguez, T.A. Razo-Lazcano, C. Solans, Green synthesis of silver nanoparticles in oil-in-water microemulsion and nanoemulsion using geranium leaf aqueous extract as a reducing agent, Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 60–67.
- Veisi, S. Azizi, P. Mohammadi, Green synthesis of the silver nanoparticles mediated by Thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water, J. Clean. Prod. 2018, 170,1536–1543.
American Cancer Society. Cancer Facts & Figures 2015; American Cancer Society: Atlanta, GA, USA, 2015.

This work is licensed under a Creative Commons Attribution 4.0 International License.









