Evaluation of the Effects of Salts on the Functional Properties of Baobab (Adansonia Digitata) Seed Flour
Habibat Omolara Adubiaro1, Bolanle Morayo Babalola1*, Abdul Ademola Olaleye2, Eunice Moriyike Ogunbusola3, Toibudeen Adesegun Sanni3 and Ibukun Omolade Arogundade1
1Industrial Chemistry Department, Federal University Oye Ekiti, Ekiti State. Nigeria
2Chemistry Department, Federal University Dutse, Jigawa State. Nigeria
3Food Technology Department, Federal University Oye Ekiti, Ekiti State. Nigeria
Corresponding Author Email: bolamorayo@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/350619
Article Received on : 23-07-19
Article Accepted on : 11-11-19
Article Published : 27 Dec 2019
Evaluation of the effects of salts on the functional properties of Adansonia digitata seed flour was investigated. Sodium chloride (NaCl), calcium chloride (CaCl2), potassium chloride (KCl), sodium ethanoate (CH3COONa) and sodium nitrate (NaNO3) salts were the salts used to carry out the investigation. The results obtained revealed that the 18% least gelation concentration recorded with distilled water was improved in the presence of salt solutions to values from 8% and 16%. Results for water absorption capacity showed a decrease from 220 in distilled water to between 136 and 220 when salt solutions were used. An increase in foaming capacity from 12.4 in the absence of salt, up to values between 24.2 and 114.4 in the presence of salt was observed. The presence of NaCl on Adansonia digitata seed flour recorded the lowest foaming stability while CH3COONa recorded the highest values; from the result it was observed that the type of salt used and its concentration had a great impact on the variation of protein solubility of Adansonia digitata seed flour with solutions of different pH.
KEYWORDS:Baobab; Salts Effect; Protein Solubility; Gelation Capacity; Foaming Capacity and Stability
Download this article as:| Copy the following to cite this article: Adubiaro H. O, Babalola B. M, Olaleye A. A, Ogunbusola E. M, Sanni T. A, Arogundade A. I. O, Evaluation of The Effects of Salts on the Functional Properties of Baobab (Adansonia Digitata) Seed Flour. Orient J Chem 2019; 35(6). |
| Copy the following to cite this URL: Adubiaro H. O, Babalola B. M, Olaleye A. A, Ogunbusola E. M, Sanni T. A, Arogundade A. I. O, Evaluation of The Effects of Salts on the Functional Properties of Baobab (Adansonia Digitata) Seed Flour. Orient J Chem 2019; 35(6). Available from: https://bit.ly/2ZrwYh7 |
Introduction
Adansonia digitata is popularly referred to as Baobab; it belongs to the family, Bombacaceae, and a member of Malvaceae sub-family of which there are about 44 species already reported in literature. The tree is commonly found in Arid and Semi-arid zones of America and some areas such as Botswana, Mozambique, South Africa and Namibia; while Asia and many other African countries are not left out1. The fruits of the tree is a large capsule in the shape of an egg (>120mm); usually embedded with yellowish-brown hairs. Each fruit can be described as being segmented into three layers: an outer woody and hard shell, followed by a layer of dry and powdery substance which act as cover for the third layer which is the hard, black kidney-shaped seed.
In Nigeria, a particular local inhabitant of Michica in Adamawa State found the pulp covering the seeds to be very useful in local condiments referred to as ‘daddawa Higgi’ and fura-de-nono’.2 Even though the pulp covering the seed is used to prepare the local drink, the latter are thrown away,so the rich harvest of these seeds waste from year to year.
The importance attributed to the functional properties of seed flour such as foaming ability, gelation, solubility and emulsifying properties, cannot be under estimated because they are the intense physico-chemical characteristics which have the ability to change the activity of food systems while processing or during the period of storage.3 It has been reported that the water content (total quantity of water) of the protein system at certain water activity values may reduce or increase the preferential binding of water to the proteins.4,5 The concentration of salts in many foods have been estimated to be between 0.2 M to 0.3 M, thus it becomes important to evaluate the effect of salts on some food properties.
This work aims at evaluating the effects of salts of various concentrations on some functional properties of Adansonia digitata seed flour; it is expected to reveal the effects of some salts such as KCl, CaCl2, NaCl, NaNO3 and CH3COONa on the functional properties of Adansonia digitata seed flour. Results obtained from this research work will provide more knowledge on the necessary conditions for processing the seed flour in order to improve its use as functional ingredients in the food system.6
Materials and Methods
Materials
Seeds of Adansonia digitata were collected from Fulani women who used the pulp for making ‘Fura-de-nono’ drink around Ahmadu Bello University Zaria, in the northern part of Nigeria. The shell of the sun-dried samples was removed and the samples were powdered and packed in polythene bags at 4OC until when needed for use.
Analytical Grade of NaCl, CaCl2, KCl, CH3COONa and NaNO3 from which different concentrations of 0.5, 1.0, 2.0, 5.0, 10.0, 15.0 and 20.0 (w/v) each were used to prepare the salt solutions.
Methods
Protein solubility with different salt concentrations
The dependence of the flour protein solubility with respect to pH was determined by using salt solution-flour suspensions and distilled water-flour systems as described by Nnadozie et al., (2015)7. In which a suspension of 1 gram of the prepared seed flour with 50cm3 of either distilled water or each of the salt solutions is stirred on a laboratory magnetic stirrer under room temperature (25oC) for 5 min.
0.1M HCl or 0.1M NaOH was engaged to change the pH of the resulting solutions to those desired values. After pH adjustment was done, the samples were centrifuged at 4000 rpm for 30 min and the supernatant was collected to determine its nitrogen content by micro-kjeldahl method8. The protein solubility was expressed as percentage nitrogen content of the sample multiplied by the 6.25 factor.
Foaming Capacity and Foaming Stability
The method by Coffmann and Garcia9 was employed to determine the foaming capacity and foaming stability of the flour. In this method, a certain quantity (1 gram) of the Adansonia digitata sample was weighed, dissolved and homogenized in 100 ml of each of the different salt solutions for 1 min using a Kenwood food blender until homogenization of the mixture was achieved. At the end of the 1 min homogenization, the mixture was immediately poured into a 100 cm3 measuring cylinder. The foaming capacity of the seed flour was determined as the foam volume 30 seconds after the homogenization was stopped (i.e. immediately it was poured into a measuring cylinder) while the foam volume that remained after 0, 30, 60, 90, 120, 150, 180 and 300 minutes is its foaming stability. The foaming stability is expressed as a percentage of the initial foam volume.
Water Absorption Capacity
A two grams portion of the flour sample was added to either 20 ml of distilled water or different salt concentrations, the suspension was thoroughly mixed and shaken on a rotary shaker at room temperature for a period of 30 minute and then centrifuged at 4000 rpm for 30 min10. The amount of water absorbed by Adansonia digitiata protein was calculated as amount of water (gram) held by 1 gram of protein sample and this is defined as the absorption capacity of the sample. The volume of water absorbed is converted to gram by assuming the density of water to be 1.000 g/ml.
Gelation Capacity
Method employed for determination of Gelation Capacity is as described in AOAC11 where 2-20% (w/v) suspensions were prepared. From each of the suspensions (distilled water/flour and salt solution/flour), five millilitres were transferred into a test tube and heated in boiling water bath for one hour. After which it was rapidly cooled in a cold-water bath, further cooling was carried out at 4±1oC for a period of two hours. The least gelation concentration was thereby determined as the sample which did not fall or slip from the test tube when it was inverted.
Results and Discussion
Gelation Concentration
The lowest gelation concentration (LGC) values for all the considered salts are presented in Table 1. The value recorded for distilled water (recorded as salt free) is 18%.
A decrease in LGC values between 8% and 18% was observed with the addition of salts; the value obtained have a direct relation to the type and concentration of salt under consideration. In the Table, the ability of Adansonia digitata seed flour to form gel (gel forming ability) was shown to be higher at higher concentration and in the presence of CaCl2. Literature by researchers who had done similar work, Akubor and Adedeji (2016)12 using locust bean pulp and Berhanu and Amare (2013)13 who used deffated brebra seed flour reported similar observation in their works on these different plants’ protein.The low value of LGC will be an advantage for using Adansonia digitata as an additive in gel formation in food products where this is desirable and also it will find good usage in curd production.
Table 1: The lowest gelation concentration of Baobab (Adansonia digitata) seed flour obtained from various concentrations of the salts
|
Percentage concentration of Lowest Gelation |
|||||
| % salt conc. | NaCl | CaCl2 | KCl | CH3COONa |
NaNO3 |
|
0.0 |
18 | 18 | 18 | 18 | 18 |
| 0.5 | 16 | 12 | 14 | 14 |
16 |
|
1.0 |
14 | 12 | 14 | 14 | 16 |
| 2.0 | 14 | 8 | 10 | 14 |
14 |
|
5.0 |
12 | 8 | 10 | 12 | 10 |
| 10.0 | 14 | 10 | 12 | 12 |
12 |
|
15.0 |
16 | 12 | 14 | 16 | 12 |
| 20.0 | 16 | 12 | 14 | 16 |
12 |
Water Absorption Capacity
The values for the water absorption capacity of Adansonia digitata seed flour in the different salts concentrations is shown in Table 2. From this table, 220% was found to be its water absorption capacity in distilled water; this value was found to be higher than the values reported for some other flours. Albizia lebbeck flour have 169.90%14, Triticum durum flour 140.63%15, Kariya kernel flour 124.3716, it was lower than that of cowpea which is 246%17.
Table 2: Water absorption capacity of Baobab (Adansonia digitata) seed flour
|
Water Absorption Capacities (%) |
|||||
| % salt conc. | NaCl | CaCl2 | KCl | CH3COONa |
NaNO3 |
|
0.0 |
220 | 220 | 220 | 220 | 220 |
| 0.5 | 170 | 168 | 164 | 211 |
180 |
|
1.0 |
162 | 154 | 162 | 210 | 174 |
| 2.0 | 160 | 148 | 178 | 182 |
170 |
|
5.0 |
150 | 142 | 156 | 164 | 162 |
| 10.0 | 148 | 136 | 154 | 160 |
222 |
|
15.0 |
208 | 184 | 198 | 202 | 236 |
| 20.0 | 412 | 268 | 210 | 234 |
340 |
It is also revealed in Table 2 that there is a continuous decrease in water absorptivity of the flour as salt concentration increases up to 15%, that is, the water absorption capacity of Adansonia digitata decreased through-out the range of salts concentrations (except at 20%) used. The decrease observed may result from masking of charges as salt concentration increases which lead to a reduction in electrostatic interaction and hydration but leads to an increase in hydrophobic interaction18.The result showed that with NaNO3, an increased in water absorptivity was observed from 10% salt concentration to 20%. From the table, it is observed that the value of water absorptivity varies depending on the type of salt used, this could be because the anion and cation involved determine the effect of salts on water absorptivity19.
Foaming Capacity and Stability
The foaming capacity presented in Table 3, shows the values obtained for the various concentrations for all the salts used in this work. The result showed that the foaming capacity of Adansonia digitata seed flour treated with salt solutions were all higher than the values obtained in the distilled water suspension. Values as high as (12.4-68.4%), (12.4-56.8%) and (12.4-114.4%) were respectively observed in the presence of NaCl, CaCl2 and KCl but lower values between (12.4-48.4%) and (12.4-50.4%) were observed in CH3COONa and NaNO3 respectively.
Table 3: Foaming Capacity at zero hour
|
Foaming Capacity at zero hour |
|||||
| % salt conc. | NaCl | CaCl2 | KCl | CH3COONa |
NaNO3 |
|
0.0 |
12.4 | 12.4 | 12.4 | 12.4 | 12.4 |
| 0.5 | 26.8 | 24.2 | 32.4 | 22.6 |
28.4 |
|
1.0 |
32.4 | 26.4 | 36.8 | 24.8 | 30.4 |
| 2.0 | 34.6 | 30.2 | 42.3 | 30.6 |
36.2 |
|
5.0 |
42.3 | 34.6 | 48.6 | 32.4 | 38.6 |
| 10.0 | 56.8 | 48.6 | 90.4 | 44.2 |
48.0 |
|
15.0 |
68.4 | 56.8 | 110.2 | 48.4 | 50.4 |
| 20.0 | 60.2 | 52.2 | 114.4 | 42.4 |
46.2 |
From the Table, it is shown that the type of salt affects the foaming capacity of the flour and we also observed that, there is an increase in the foaming capacity irrespective of the salt used when the salt concentration is between 0.5 to 15.0%. The observed increase may be as a result of the fact that the surface viscosity and rigidity of protein films reduce in the presence of salt whereas the presence of salt leads to increase spreading rate and thus weakening inter-peptide attraction and increasing volume for certain proteins 6. High foaming capacity enhance the functionality of Adansonia digitata seed flour when it is used in situations where foaming is a desirable property, for instance, in the production of cakes and whipped toppings. The foaming capacity values were observed to be highest in the presence of KCl while NaCl has the next higher values, which agrees with that of Quinoa flour20.
The values obtained for the foaming stability with different salts are shown in Table 4 to 8, from these tables the decrease in foaming stability follows the order: NaCl: (rate of 0.20 to 0.27min-1)<KCl: (rate of 0.22 to 0.26 min-1) <CaCl2: (rate of 0.25 to 0.27 min-1) <NaNO3 (rate of 0.28 to 0.30 min-1) <CH3COONa:(rate = 0.27 to 0.33 min-1).
Table 4: % Foaming stability ofAdansonia digitata in different concentrations of NaCl solution
|
Time (min) / Salt conc. (%) |
0.0 | 0.5 | 1.0 | 2.0 | 5.0 | 10.0 | 15.0 | 20.0 |
| 0.0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
100 |
|
30 |
60.44 | 2.6 | 48.5 | 56.8 | 58.2 | 60.2 | 60.0 | 61.1 |
| 60 | 28.8 | 33.5 | 28.6 | 32.3 | 40.8 | 45.6 | 46.4 |
50.2 |
|
90 |
28.6 | 30.4 | 28.6 | 30.5 | 40.8 | 42.3 | 44.4 | 48.2 |
| 120 | 10.4 | 30.4 | 24.6 | 30.5 | 38.6 | 40.0 | 32.6 |
46.4 |
|
150 |
10.2 | 30.4 | 24.6 | 30.0 | 38.6 | 40.4 | 32.6 | 46.4 |
| 180 | 10.2 | 26.2 | 18.2 | 28.2 | 30.2 | 38.8 | 32.6 |
46.4 |
|
300 |
8.4 | 26.2 | 18.2 | 28.2 | 30.2 | 38.8 | 30.0 | 40.0 |
| Mean | 32.1 | 40.0 | 36.4 | 42.1 | 47.2 | 50.7 | 49.0 |
55.1 |
|
SD |
32.6 | 24.8 | 27.4 | 25.3 | 23.0 | 21.1 | 22.9 | 19.1 |
| CV % | 101 | 62 | 75 | 60 | 49 | 42 | 47 |
37 |
|
Rate/min |
0.31 | 0.25 | 0.27 | 0.24 | 0.23 | 0.20 | 0.23 |
0.20 |
Table 5: Foaming stability of Adansonia digitata in different concentrations of KCl solution
|
Time (min) / Salt conc. (%) |
0.0 | 0.5 | 1.0 | 2.0 | 5.0 | 10.0 | 15.0 | 20.0 |
| 0.0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
100 |
|
30 |
60.4 | 52.3 | 54.8 | 60.2 | 62.8 | 68.4 | 60.6 | 60.2 |
| 60 | 28.8 | 34.2 | 36.8 | 38.4 | 38.0 | 38.2 | 40.0 |
38.2 |
|
90 |
28.6 | 34.2 | 35.6 | 36.6 | 36.4 | 38.0 | 36.2 | 36.6 |
| 120 | 10.4 | 34.0 | 35.6 | 33.2 | 36.0 | 36.2 | 34.0 |
32.4 |
|
150 |
10.2 | 32.2 | 34.8 | 33.2 | 35.2 | 36.2 | 32.0 | 30.0 |
| 180 | 10.2 | 32.2 | 34.8 | 30.0 | 34.4 | 32.8 | 30.0 |
28.0 |
|
300 |
8.4 | 30.0 | 34.8 | 28.2 | 30.0 | 28.4 | 26.4 | 22.2 |
| Mean | 32.1 | 43.6 | 46.0 | 45.0 | 46.6 | 47.3 | 44.9 |
43.5 |
|
SD |
32.6 | 23.8 | 22.8 | 24.4 | 24.0 | 24.5 | 24.6 | 25.5 |
| CV % | 101 | 55 | 50 | 54 | 52 | 52 | 55 |
59 |
|
Rate/min |
0.31 | 0.23 | 0.22 | 0.24 | 0.23 | 0.24 | 0.25 |
0.26 |
Table 6: Foaming stability of Adansonia digitata in different concentrations of CaCl2 solution
|
Time (min) / Salt conc. (%) |
0.0 | 0.5 | 1.0 | 2.0 | 5.0 | 10.0 | 15.0 | 20.0 |
| 0.0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
100 |
|
30 |
60.4 | 62.6 | 48.8 | 48.6 | 46.0 | 40.0 | 40.0 | 38.2 |
| 60 | 28.8 | 32.4 | 36.8 | 34.8 | 32.6 | 30.0 | 30.8 |
30.0 |
|
90 |
28.6 | 32.4 | 32.8 | 34.0 | 30.8 | 28.6 | 28.8 | 30.0 |
| 120 | 10.4 | 32.4 | 32.4 | 32.6 | 32.6 | 28.6 | 28.0 |
26.0 |
|
150 |
10.2 | 28.8 | 30.3 | 32.6 | 30.0 | 28.6 | 28.0 | 26.0 |
| 180 | 10.2 | 26.4 | 30.0 | 30.0 | 28.2 | 26.4 | 26.0 |
24.0 |
|
300 |
8.4 | 18.4 | 26.4 | 22.2 | 20.2 | 22.2 | 20.4 | 20.2 |
| Mean | 32.1 | 41.6 | 42.2 | 42.0 | 40.0 | 38.1 | 37.8 |
36.6 |
|
SD |
32.6 | 26.9 | 24.3 | 24.6 | 25.3 | 25.5 | 25.7 | 26.1 |
| CV % | 101 | 65 | 58 | 59 | 63 | 67 | 68 |
71 |
|
Rate/min |
0.31 | 0.27 | 0.25 | 0.26 | 0.27 | 0.26 | 0.27 |
0.27 |
Table 7: Foaming stability of Adansonia digitata in different concentrations of CH3COONa
|
Time (min)/ salt conc. (%) |
0.0 | 0.5 | 1.0 | 2.0 | 5.0 | 10.0 | 15.0 | 20.0 |
| 0.0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
100 |
|
30 |
60.4 | 32.4 | 28.6 | 26.2 | 24.4 | 22.0 | 20.0 | 18.4 |
| 60 | 28.8 | 30.0 | 24.8 | 24.4 | 20.0 | 20.0 | 20.0 |
0.0 |
|
90 |
28.6 | 30.0 | 22.6 | 22.2 | 18.4 | 16.4 | 16.4 | 0.0 |
| 30 | 10.4 | 28.2 | 20.2 | 18.8 | 16.4 | 16.2 | 16.4 |
0.0 |
|
150 |
10.2 | 28.2 | 20.2 | 16.8 | 14.4 | 14.2 | 14.2 | 0.0 |
| 180 | 10.2 | 20.0 | 18.4 | 14.2 | 12.8 | 12.8 | 12.6 |
0.0 |
|
300 |
8.4 | 14.2 | 18.4 | 12.2 | 10.2 | 10.2 | 10.2 | 14.8 |
| Mean | 32.1 | 35.4 | 31.7 | 29.4 | 27.1 | 26.5 | 26.2 |
35.0 |
|
SD |
32.6 | 26.8 | 27.8 | 29.0 | 30.0 | 30.0 | 30.0 | 19.1 |
| CV % | 101 | 76 | 88 | 99 | 111 | 113 | 115 |
236 |
|
Rate/min |
0.31 | 0.29 | 0.27 | 0.29 | 0.30 | 0.30 | 0.30 |
0.33 |
Table 8: Foaming stability of Adansonia digitata in different concentrations of NaNO3
|
Time (min)/ salt conc. (%) |
0.0 | 0.5 | 1.0 | 2.0 | 5.0 | 10.0 | 15.0 | 20.0 |
| 0.0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
100 |
|
30 |
60.4 | 34.6 | 32.8 | 36.8 | 36.8 | 36.8 | 32.8 | 30.0 |
| 60 | 28.8 | 30.8 | 28.8 | 26.4 | 30.2 | 32.6 | 32.8 |
30. |
|
90 |
28.6 | 28.8 | 26.2 | 26.2 | 28.4 | 28.4 | 30.4 | 30.0 |
| 120 | 10.4 | 28.8 | 26.2 | 26.2 | 28.4 | 28.4 | 30.2 |
28.2 |
|
150 |
10.2 | 20.2 | 26.2 | 24.2 | 26.2 | 28.2 | 30.2 | 28.2 |
| 180 | 10.2 | 20.2 | 26.2 | 24.2 | 26.2 | 26.2 | 26.2 |
26.2 |
|
300 |
8.4 | 10.6 | 15.2 | 14.4 | 12.4 | 12.4 | 12.4 | 12.4 |
| Mean | 32.1 | 34.3 | 35.2 | 35.0 | 36.1 | 36.6 | 37.0 |
35.6 |
|
SD |
32.6 | 27.6 | 26.6 | 27.0 | 26.7 | 26.5 | 26.4 | 26.7 |
| CV % | 101 | 80 | 76 | 77 | 74 | 72 | 71 |
75 |
|
Rate/min |
0.31 | 0.30 | 0.28 | 0.29 | 0.29 | 0.29 | 0.29 |
0.29 |
After 2 hours without the addition of any salt, the foaming stability value of 10.4% was obtained for the Adansonia digitata seed flour. This value is higher than the value of Casssia hirsutta 8.0% and that of Canavalia ensiformis 3.0%21 and lower than that of hull African yam bean seeds flour whose value is between 43.3 – 42.5%22 and cowpea flour 91.0%23 during the same duration. Foaming stability being an important property in flour and since to a large extent the success of a whipping agent depends on its ability to retain the whip for as long as possible, Adansonia digitata seed flour will be better in the presence of salts and best using either NaNO3 and CH3COONa, and it also suggest that the flour obtained from this seed would be appropriate for food formulation where foaming stabilities is necessary.
Protein Solubility
Results for the variation of protein solubility with pH (with and without salts) are shown in Fig 1 to 5. The figures show that pH and salt concentrations affect the variations in protein solubility of Adansonia digitata. For this study, the solubility curve showed a minimum around pH 5 when no salt was added to the seed flour. Fig 1 and 2 revealed the minimum solubility occurred at about pH 6 when 0.5% to 10% NaCl and CaCl2 were added. In Fig 3, the pH 5.0 observed at zero concentration of KCl shifted to pH 4 when 10% of the salt was added.
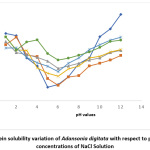 |
Figure 1: Protein solubility variation of Adansonia digitata with respect to pH in different concentrations of NaCl Solution Click here to View Figure |
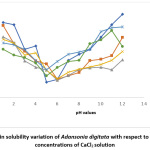 |
Figure 2: Protein solubility variation of Adansonia digitata with respect to pH in different concentrations of CaCl2 solution Click here to View Figure |
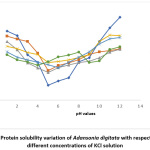 |
Figure 3: Protein solubility variation of Adansonia digitata with respect to pH in different concentrations of KCl solution Click here to View Figure |
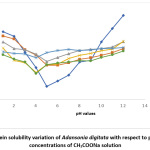 |
Figure 4: Protein solubility variation of Adansonia digitata with respect to pH in different concentrations of CH3COONa solution Click here to View Figure |
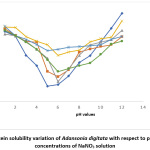 |
Figure 5: Protein solubility variation of Adansonia digitata with respect to pH in different concentrations of NaNO3 solution Click here to View Figure |
The result in Fig 4 showed the effects of CH3COONa on Adansonia digitata seed flour, the minimum pH 5 at zero concentration of CH3COONa was changed to pH 3 for 2%, 5% and 10% salt concentrates. The result when NaNO3 salt was used is shown in Fig 5, the minimum solubility at pH 5when no salt was present changed to pH 6 for all the concentrations of this salt tested. A general observation from this work is that the protein of the seed flour was more soluble in the acid region of pH when any of the five salts used in this work was present than when none of the salt is present; whereas the opposite was observed in the basic region, lower solubility were obtained in the presence of any of the salts. This observation is similar to what was reported in Conophor nut, legume protein and benniseed flour24,25 and 26. The degree of hydrophobicity of protein molecules and its level of hydration affect the solubility of protein 5. High concentration of salts, more precisely the presence of CaCl2 enhanced the gel forming property of Adansonia digitata seed. The variation in water absorption capacity (decrease or increase) is influenced by the type and concentrations of the salt used. The presence of KCl produced the highest foaming capacity, this was followed by NaCl. The foaming stability will be better in the presence of salts and best using either NaNO3 or CH3COONa.The protein solubility also depended on the pH and concentration of salts. Thus, it can be concluded that salts affected the functional properties of Adansonia digitata seed flour.
Conflict of Interest
We declare that there is no conflict of interest
Acknowledgement
Authors are grateful to the Chemistry Department of Ekiti State University for providing necessary facilities for this work
References
- Kamatou G. P., Vermaak I., Viljoen A. M. An updated Review of Adansonia digitata: a commonly important African tree. Afri. J. Bot, 201177: 908 – 919
- Nkafamiya I. I., Manji A. J., Modibbo U. U. and Umaru H. A. (2006). Biochemical evaluation of Cassipourea congoensis (Tunti) and Nuclealatiflokia (Luzzi) fruits. Afri J. of Biotech.2006 6 (19): 2461 – 2463
- Oladele A. K and Aina J.O. Chemical Composition and Functional Properties of flour produced from two varieties of tigernut (Cyperus esculentus) J. Biotech 2007 6:2473 – 2476
- Ahmed S. H., Babiker E. E., Ahmed Muhammed I. A., Ehayeb M.M.. Ahmed S. O. and Faridullah. Effect of Sodium Chloride Concentration on the functional properties of selected legume flours.African Journal of Food, Agriculture, Nutrition and Development 2012. 12 (6): 6700 – 6712
- Serema M., Surangna J and Anilcumar A. Effect of sprouting and cooking processes of moth bean (Vigna aconitifolia) seed flour J Fd Sci and Tech201956: 1022 – 1024
- Fagbemi, T. N., Oshodi, A. A. and Ipinmoroti, K. O. Effect of processing and salts on some functional properties of cashew nut (Anarcadium occidentale) flour. Fd, Agric. and Env 2004. 2 (2): 121-128
- Nnadozie, E.F., Kelechi, A.J. and Deborah, O.Effects pH and NaCl on the Protein Solubility, Emulsifying and Foaming Properties of Germinated and Ungerminated Melon (Colocynthis citrullus) Seed Flour. J. Food Sci. Nutr. 20154(2): 173-177.
- Arab, E.A., Helmy, I.M.F. and Bareh, G.F. Nutritional evaluation and functional properties of chickpea (Cicer arietinum) flour and the improvement of spaghetti produced from it. Am J Sci.,2010. 6 (10): 1055-1072.
- Coffman, C. W. and Garcia, V. C. Functional properties and amino acid content of a protein isolate from mung bean flour. Fd. Technol.2009.12: 437 – 438.
- Ogungbenle H.N. Effects of salt concentrations on the functional properties of some legume flours. Pakis J. of Nutri 2008 7:453 – 458.
- Adubiaro H. O., Olaofe O. and Sanni T. A. Effects of Salts on the Functional Properties of Daniellia oliveri Seed Flour. FUW Trends In Science and Technology Journal. 2018.3(1): 329 – 334
- Official methods of Analysis, 21st edn, Washington D. C. 2010 Association of Analytical Chemists.
- Akubor P .I. and Adedeji E. O. Effects of pH and Sodium Chloride Concentration on the functional properties of locust bean pulp flour. FUW Trends In Science and Technology Journal.2016. 1 (2): 344 – 347.
- Berhanu A. and Amare G. Effects of Salt (NaCl) Concentrations on the Functional properties of Deffated Brebra (Millettia ferruginea) seed flour. Middle – East Journal of Scientific Research. 2013. 13 (7): 889 – 897.
- Adubiaro, H.O., Olaofe, O. and Akintayo, E. T. Effects of salts on the functional properties of Albizia lebbeck seed flour. Electro J. of Enviro, Agric. and Fd. Chem. 2009 8(8): 692-703.
- Adeyeye, E. I. and Aye, P. A. Chemical Composition and the effect of salts on the food properties of Triticum durum whole meal seed flour. Pakis. J. of Nutr. 2005 4(3): 187-196.
- Adebayo, W. A., Ogunsina, B. S. and Gbadamosi, S. O. Some physico – chemical and Functional properties of Kariya (Hildegardia baterii) kernel flours. Ife Journal of Science. 2013. 15 (3): 477 – 488.
- Olaofe, O., Adediran G. O. and Umar, Y.O. The effect of nematicides on the nutritive value and functional properties of Cowpea seed (Vignaungui calatawalp). Fd. Chem.1993 46: 337-341.
- Sorgentini, D. A. and Wagner, J. R. Comparative study of Foaming Properties of Whey and Isolate Soybean Proteins. Food Research International. 2002. 35: 721 – 729.
- Adegunwa M. O., Adebowale A. A. and Solano E. O. Effect of thermal processing on the biochemical composition, antinutrition and functional properties of Beniseed (Sesamum indicum) flour. American Journal of Biochemistry and Molecular Biology. 2012. 2 (3): 175 – 182
- Ogungbenle H. N., Oshodi A. A. and Oladimeji M.O. The Proximate and Effect of Salt Applications on some Functional Properties of Quinoa (Chenopodium quinoa) flour. Pakistan Journal of Nutrition 8 (1): 49 – 52.
- Ojo M. A. and Ade-Omowaye B. I. O. Some Functional and Physical Properties of Selected Underutilised Hard- To- Cook Legumes In Nigeria. American Journal of Food Science and Nutrition. 2015. 2 (5): 73 – 81
- Adeyeye E. I., Oshodi, A. A. and Ipinmoroti. Functional properties of some varieties of African yam bean (Sphenostylis stenocarpa) flour. Int. J. Food Sci.Nutri. 1994. 45: 115 – 126
- Audu S. S. and Aremu M.O. Nutritional composition of raw and processed pinto bean (Phaseolus vulgaris L.) grown in Plateau State, Nigeria. Fd Agric. Environ. 2011 9: 72 – 80.
- Saka O. G., Sumbo H. A and Rotimi E. A. Amino acid profile, protein digestibility, thermal and functional properties of Conophor nut (Tetracarpidium conophorum) defatted flour, protein concentrate and isolate. J. of Fd Sci and Tech. 2012 47: 731 – 739.
- Masood S. B and Rizwana B. Nutritional and Functional properties of some promising legumes protein isolate. Pakis J. of Nutri. 9: 373 – 379.
- Ogungbenle, H.N., Oshodi, A. A. and Oladimeji, M.O. Effect of salts on the functional properties of beniseed (Sesanum radiatum) seed flour. J. of Fd Sci and Nutri 2002 53: 1-5.

This work is licensed under a Creative Commons Attribution 4.0 International License.









