Docking of Some Bioactive 2-Chloro-N, N-Diphenylacetamide Derivatives on Cyclo-Oxygenase Enzyme and In-Vivo Analgesic Activity Evaluation
Arvind Kumar*, Sushil Kumar and Arun K Mishra
Drug Design Laboratory, Faculty of Pharmacy, IFTM University, Moradabad, 244001, Uttar Pradesh, India
Corresponding Author Email: akarvindsr01@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350602
Article Received on : 07-11-2019
Article Accepted on : 10-12-2019
Article Published : 18 Dec 2019
The present work was aimed to synthesize some novel 2-chloro-N,N-diphenylacetamide derivatives conjugated with various benzaldehydes in multistep synthesis and further to undergo the process of docking on COX-1 and COX-2 enzymes. All the targeted synthesized compounds were subjected to evaluation of analgesic activity using hot plate model. Sophisticate analytical techniques via infrared spectroscopy, NMR, Mass spectroscopy and elemental analysis were employed to characterize the newly synthesized compounds. All the synthesized derivatives were evaluated for their analgesic activity. The synthesized compound AKM-2 (N-(4-(diphenylamino) thiazol-2-yl)-2-((4-((3methylbenzylidene)amino)phenyl)amino)acetamide) exhibited significant analgesic response when comparison was made with diclofenac sodium as standard drug. Molecular docking study was used to discover the possibility of binding site and binding strength of new derivatives of acetamide. The present study offers insight for compound AKM-2, as a new lead compound as analgesic agent because of significant docking and similar amino acid residue. The biological activity performed by in-vivo method confirmed the same.
KEYWORDS:Prostaglandins, Nonsteroidal anti-inflammatory drugs, Molecular docking, Acetamide, Analgesic activity.
Download this article as:| Copy the following to cite this article: Kumar A. Kumar S. Mishra A. K. Docking of Some Bioactive 2-Chloro-N, N-Diphenylacetamide Derivatives on Cyclo-Oxygenase Enzyme and In-Vivo Analgesic Activity Evaluation. Orient J Chem 2019; 35(6). |
| Copy the following to cite this URL: Kumar A. Kumar S. Mishra A. K. Docking of Some Bioactive 2-Chloro-N, N-Diphenylacetamide Derivatives on Cyclo-Oxygenase Enzyme and In-Vivo Analgesic Activity Evaluation. Orient J Chem 2019; 35(6). Available from: https://bit.ly/2tnbkP1 |
Introduction
Cyclooxygenase-1 and Cyclooxygenase-2, in combination called COX enzymes, directly exert effect on Prostaglandins synthesis. Prostaglandins and associated products are produced in very small amounts majorly responsible for pain, inflammation etc. The circulation of prostaglandins does not occur in the blood with substantial concentration.1
COX enzymes are accountable for analgesic response, inflammation and other physiological action.2
Non-steroidal anti-inflammatory drugs (NSAIDs) are one of the commonly utilized medications all over the world and majorly used for cure and prevention of pain and inflammation associated with a number of pathologies. NSAIDs for example include diclofenac and tolfenamic acid etc. NSAIDS act as a blocking agent in biosynthesis of prostaglandins by inhibiting the action of COX enzyme. The cyclooxygenases (COXs), otherwise called as prostaglandin H2 synthases are bifunctional, membrane-bound enzymes that interfere and catalyze the dedicated step in prostanoid biosynthesis.3-4
In order to find out exact mechanism and fitting of bioactive compounds with receptor enzyme, docking is latest tool, being used in contemporary times.
Molecular docking is an attractive thrust area of research to know drug biomolecular relations for the rational use of basics of drug design in drug discovery. Molecular docking elucidates the drug action mechanism by placing an atom (ligand) into the limiting site of the target exact area of the protein (receptor). The information obtained from the docking study can be employed to conclude suggest the binding energy, free energy and stability of drug-receptor complex. Currently, molecular docking is utilized to calculate the tentative binding parameters of ligand-receptor complex earlier.5-7
The process of molecular docking requires data bank to identify the target with suitable PDB format and a method to plan ligand in PDB file format. There are a variety of software’s (Discovery studio, etc.,) in PDB format. These tools provide an idea of ligands related with their ability to connect with given target receptors. Molecular docking includes a pre-defined sampling of probable conformation of ligand in order to set the optimized conformation of the complex. This is generally done by scoring function of software.8
Molecular docking can also be used to calculate dissimilar binding approach of ligand. This can be used to increase more effective, selective and resourceful drug applicant.9
Previous studies over the decade have demonstrated that compounds with acetamide nucleus possess many therapeutic properties. This includes anti-viral10, antitubercular11, anticonvulsant12, antimicrobial13, antipsychotics14 and antimicrobial activities15.
Here, our study will be focused to examine the docking of synthesized compounds with reference COX-1 and COX-2 enzymes and to evaluate analgesic activity of 2-chloro-N,N-diphenylacetamide derivatives using in vivo model.
Materials and Methods
General Methods
In the present research work, Diphenylamine, toluene, chloroacetylchloride, thiourea and various substituted benzaldehyde were purchased from CDH Pvt. Ltd., New Delhi and S.D. Fine, Mumbai, India. The synthetic scheme for the synthesis is presented in scheme 1.
Purification of the synthesized compounds (AKM-1, AKM-2 and AKM-3) were performed by the recrystallization with appropriate solvent ethanol. The level of purity of newly synthesized compounds was screened by TLC method. The solvent system employed was Ethyl acetate: hexane (1:4). In order to determine the melting temperature of intermediates and compounds, standard method (open capillary tubes method) was employed and recorded as uncorrected. The FTIR spectrum was recorded by potassium bromide pellet method on FTIR spectrophotometer (8400SS Shimadzu, Japan). The 1HNMR spectrum was recorded (Broker Jeol at 300MHz) spectrophotometer in CDCl3. TMS was employed as an internal standard. The 1H chemical shifts were recorded as parts per million (ppm) downfield from TMS. The mass spectrum was obtained with Shimadzu GCMS-QP-2000 mass spectrometer. The elemental analysis was performed to quantitatively evaluate the (%) amount of Carbon, Oxygen, Hydrogen and Nitrogen on element analyser (Carlo Erba-1108).
Where,
R= 2-OH (AKM-1), 3-CH3 (AKM-2), 3-OH (AKM-3)
Scheme 1: Synthetic reaction scheme for 2-chloro-N, N-diphenyl-acetamide derivatives. The employed reagents and conditions for synthesis are presented as: a) chloroacetylchloride, toluene, reflux b) thiourea, ethyl alcohol, reflux c) chloroacetylchloride, dimethyl ketone, anhydrous potassium carbonate, reflux, 10h d) 4-amino aniline, triethyl amine, stirring, dimethyl ketone, 13h e) substituted benzaldehydes (eg. 2-hydroxy benzaldehyde,3-methyl benzaldehyde, 3-hydroxy benzaldehyde), ethanol, glacial acetic acid, reflux, 12-14 h
Synthesis Method
2-chloro-N,N-diphenyl acetamide (A)
The compound (A) was synthesized employing chloroacetylation of diphenylamine in toluene according to the reported procedure.14,16
N4,N4-diphenylthiazole-2,4-diamine (B)
The synthesized compound (A) was refluxed with thiourea in ethanol to obtain N4,N4 diphenylthiazole-2,4-diamine (B) according to reported procedure.17
2-chloro-N-(4-(diphenyl amino) thiazol-2-yl) acetamide (C)
2-chloro-N-(4-(diphenyl amino) thiazol-2-yl) acetamide (C) was synthesized by chloroacetylation reaction. Compound B was refluxed (0.1 mol) with chloroacetyl chloride (0.1 mole) in chloroform (100 ml) about 10 h. During the reaction period, the presence of potassium carbonate (anhydrous) was ensured. The reaction progress was monitored on TLC plate. Afterwards, for work out of reaction, the vacuum dryer was used to remove the excess content of solvent and prior to filtration; the residue obtained was stirred using water. The product was filtered, washed with 2% NaHCO3 and then using distilled water. Methanol was employed for recrystallization of the compound. The characterization findings of C are as follows:
IR (KBr) cm-1: 1453 (C=C str Aro); 1590 (C-C str Aro); 3053 (C-H str Aro); 2838 (C-H str Ali);1525 (C-N-C); 823 (C-S-C); 1690 (C=O str); 3265 (N-H str); 697 (C-Cl str) 1H-NMR (400 MHz, CDCl3) (ppm): 7.25-7.78; (m, 10H, aro.); 5.34 (s, 1H, CH of thiazole); 9.12 (1H, NH); 4.85 (2H, CH2), MS (FAB) [M+1]+:(m/z) 345 Elemental analysis calculate (found) C= 59.38 (59.10), H= 4.10 (4.05), O=4.65 (4.53), N=12.22 (12.24)
Synthesis of 2-((4-aminophenyl)amino)-N-(4-(diphenylamino)thiazol-2-yl)acetamide (D)
2-((4-aminophenyl)amino)-N-(4-(diphenylamino)thiazol-2-yl)acetamide (D) was prepared by continuous stirring of 0.01 mol of 2-chloro-N-(4-(diphenyl amino) thiazol-2-yl) acetamide (C) and 0.01 mol of 4-amino aniline in dimethyl ketone (100 ml) using (triethyl)-amine (0.01 mol) as a catalyst on magnetic stirrer for 13 h. The reaction progress was monitored using TLC plate. The obtained product was filtered, dried and then recrystallized from absolute ethanol.
IR (KBr) cm-1: 1455 (C=C str Aro); 1592 (C-C str Aro); 3058 (C-H str Aro); 2840 (C-H str Ali);1530 (C-N-C); 824 (C-S-C); 1693 (C=O str); 3265 (N-H str); 1H-NMR (400 MHz, CDCl3) (ppm): 7.28-7.76; (m, 10H, aro.); 5.34 (s, 1H, CH of thiazole); 9.12 (s, 1H, NH); 3.65 (s, 2H, CH2), 6.25-6.32; (m, 4H, aniline.); 6.27 (2H, NH2), MS (FAB) [M+1]+:(m/z) 416.15
Elemental analysis calculated (found) C= 66.48 (66.41), H= 5.09 (5.05), O=3.85 (3.82), N=16.85 (16.81)
General procedure for the preparation of 2-chloro-N,N-diphenyl acetamide derivatives (AKM-1 to AKM-3)
0.001mole of 2-((4-aminophenyl) amino)-N-(4-(diphenylamino) thiazol-2-yl) acetamide (D) and 0.001mole of suitable various aromatic aldehydes were dissolved in ethyl alcohol (100ml) in a 250 ml of RBF. The RBF was refluxed for 12h with catalytic quantity of 3-4 drops of glacial acetic acid (GAA). For the precipitation, the resulted mixture was cooled and reserved overnight. The completion of reaction was checked using TLC plate. The obtained precipitate of product was filtered, dried and recrystallized from absolute ethanol. In this reaction process, different benzaldehydes viz. 2-hydroxybenzaldehyde, 3-methylbenzaldehyde and 3-hydroxybenzaldehyde were used which resulted AKM-1, AKM-2 and AKM-3 respectively. The structure of all the synthesized compounds (AKM-1, AKM-2 and AKM-3) are presented in Table 1 and the findings of spectral characterization findings are presented as follows:
 |
Table 1: Physical characterization data and elemental analysis of newly synthesized compounds. Click here to View Figure |
N-(4-(diphenylamino)thiazol-2-yl)-2-((4-((2-hydroxybenzylidene)amino)phenyl)amino) acetamide (AKM-1)
IR (KBr) cm-1: 1454 (C=C str Aro); 1590 (C-C str Aro); 3050 (C-H str Aro); 2838 (C-H str Ali.);1527 (C-N-C); 823 (C-S-C); 1690 (C=O str); 3260 (N-H str); 1574 (C=N str); 1H-NMR (400 MHz, CDCl3) (ppm): 7.20-7.68; (m, 14H, aro.); 5.42 (s, 1H, CH of thiazole); 9.10 (s, 1H, NH); 3.82 (s,2H, CH2), 6.36-6.60; (m, 4H, aniline); 4.75 (s, 1H, CH); 5.12 (s,1H, OH); MS (FAB) [M+1]+:(m/z) 520.18
N-(4-(diphenylamino)thiazol-2-yl)-2-((4-((3-methylbenzylidene)amino)phenyl)amino) acetamide (AKM-2)
IR (KBr) cm-1: 1455 (C=C str Aro); 1592 (C-C str Aro); 3051 (C-H str Aro); 2835 (C-H str Ali);1524 (C-N-C); 825 (C-S-C); 1690 (C=O str); 3255 (N-H str); 1574 (C=N str); 1H-NMR (400 MHz, CDCl3) (ppm): 7.25-7.73; (m, 14H, aro.); 5.44 (s, 1H, CH of thiazole); 9.12 (s, 1H, NH); 3.60 (s, 2H, CH2), 6.40-6.60; (m, 4H, aniline); 4.78 (s, 1H, CH); 2.30 (s, 3H, CH3); MS (FAB) [M+1]+:(m/z) 518.20
N-(4-(diphenylamino)thiazol-2-yl)-2-((4-((3-hydroxybenzylidene)amino)phenyl)amino) acetamide (AKM-3)
IR (KBr) cm-1: (C=C str Aro); 1590 (C-C str Aro); 1450 3055 (C-H str Aro); 2840 (C-H str Ali);1520 (C-N-C); 827 (C-S-C); 1688 (C=O str); 3258 (N-H str); 1576 (C=N str); 1H-NMR (400 MHz, CDCl3) (ppm): 7.35-7.81; (m, 14H, aro.); 5.42 (s, 1H, CH of thiazole); 9.10 (s, 1H, NH); 3.82 (s, 2H, CH2), 6.36-6.60; (m, 4H, aniline); 4.68 (s, 1H, CH); 5.32 (s, 1H, OH), MS (FAB) [M+1]+:(m/z) 520.18
Molecular Modeling Studies
PDB files of target proteins (Cyclooxygenase-1 and Cyclooxygenase-2) were downloaded from protein data bank (PDB: 2oyu and PDB: 1PXX). Docking studies have been carried out for 2-chloro-N,N-diphenylacetamide derivatives with analgesic activity. These compounds were evaluated to acess the analgesic activity by inhibiting Cyclooxygenase-1 and Cyclooxygenase-2 enzyme. Two dimensional (2D) structures of newly synthesized molecules were drawn at ChemDraw/ChemSketch editor. These 2D structures were saved in .mol file format. The 2D structures were further converted to 3D structure and their structural geometry was optimized to lowest energy state. Finally, stable 3D structures were saved in pdb format. After preparation of ‘pdb’ files of ligand compounds as well as protein structures, it was subjected for docking study. AutoDock Vina software (Trott and Olson, 2010) was used to carry out docking study, which simulates molecular interaction studies of ligand and protein, through Lamarckian genetic algorithm. AutoDock Vina has higher accuracy as well as computational performance over traditional AutoDock software. Whole protein structure was considered for docking. For each docking study, best generated pose was used for analysis. Each docking study resulted into negative binding affinity values containing unit of kilo-calorie per mol (kcal/mol). The findings are presented in Table 2.
Table 2: Binding energy and Interaction Residue of Ligands.
|
Sr. No. |
Ligands | Binding energy (kcal/mol) | Interaction residues | |||
|
Cyclooxygenase-1 |
Cyclooxygenase-2 | Cyclooxygenase-1 |
Cyclooxygenase-2 |
|||
| 1 | Diclofenac | -7.4 | -8.4 | TYR |
Pi-pi: TRP |
|
|
2 |
AKM-1 | -8.8 | -7.4 | ARG | Pi-pi: PHE, TYR H bond: GLY, TYR, ARG |
|
| 3 | AKM-2 | -9.0 | -9.1 | ARG, TYR |
TRP |
|
|
4 |
AKM-3 | -9.0 | -8.9 | ARG |
– |
|
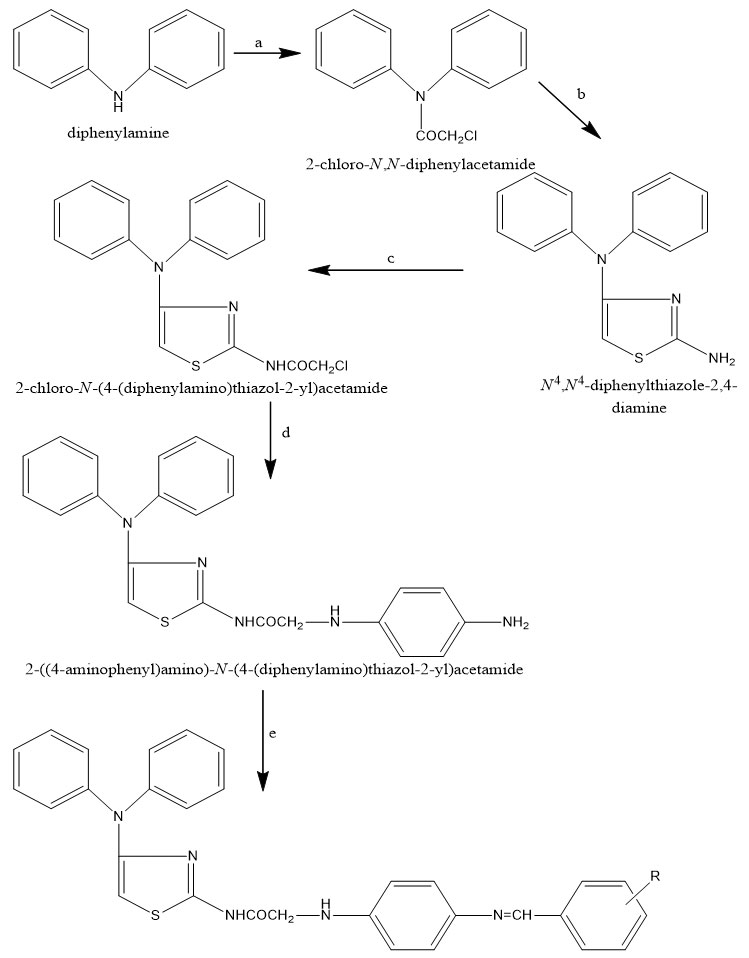
The molecular docking of all the three synthesized compounds and standard drug (diclofenac) is presented in fig. 2-5.
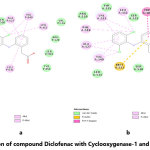 |
Figure 2: Interaction of compound Diclofenac with Cyclooxygenase-1 and Cyclooxygenase-2 Click here to View Figure |
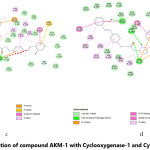 |
Figure 3: Interaction of compound AKM-1 with Cyclooxygenase-1 and Cyclooxygenase-2 Click here to View Figure |
 |
Figure 4: Interaction of compound AKM-2 with Cyclooxygenase-1 and Cyclooxygenase-2 Click here to View Figure |
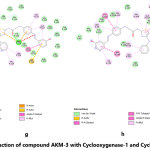 |
Figure 5: Interaction of compound AKM-3 with Cyclooxygenase-1 and Cyclooxygenase-2 Click here to View Figure |
Biological Evaluation
Experimental Animals
Male and female wister albino rats refer from some research paper and rats (140–200 g) were used throughout the study. The rats were procured from the Institutional Animal House, IFTM University, Moradabad, India. The rats were kept and maintained as per provision of The Committee for the Purpose of control and supervision of experiments on animals (CPCSEA) guidelines with ethical clearance (2016/837ac/Ph.D./10.).
Preparation of Test Compounds
The suspension of standard drug and test samples were made in 10% Tween 20. Group I, used as control received 10% tween 20, through oral route. Animals of group II were termed as standard group and were treated with the suspension of diclofenac sodium with a dose of 50 mg/kg. Other test group animals were treated with the suspension of with a dose of 200 mg/kg of newly synthesized compounds (AKM-1 to AKM-3).
Screening of Analgesic action
Eddy’s Hot Plate Method
All the target synthesized compounds (AKM-1 to AKM-3) were evaluated for their analgesic response using Eddy’s hot plate model. The rats were divided into five groups (n=6). The hot plate was used in the present study, having an electrically heated face. Tested animals were placed one by one on the hot plate, conserved at a stable heat 55°C, time until their paw licking or jump reaction of animals was note down by stop watch. In order avoid the paws injury of rats, 15 seconds cut-off time was used as uppermost analgesic response. Diclofenac sodium (50 mg/kg) was used as reference drug for judgment. After the administration of reference drug and test compounds, the response time period for every rat was recorded at 0, 60, 120 and 180 m.18-20 The findings of analgesic response are presented in Table 3.
Table 3: Analgesic effect of newly synthesized derivatives
|
Group |
Administered dose (mg/kg) | Analgesic effect Reaction time (min., mean ± SEM) | |||
| 0 m | 60 m | 120 m |
180 m |
||
|
Control (10% tween 20) |
5 ml/kg | 2.42±0.10 | 2.48±0.13 | 2.50±0.12 | 2.47±0.10 |
| Diclofenac sodium | 50 mg/kg | 4.82±0.11** | 5.60±0.15*** | 7.13±0.20*** |
6.72±0.19*** |
|
AKM-1 |
200 mg/kg | 2.96±0.12ns | 3.21±0.15** | 3.94±0.17** | 3.14±0.16** |
| AKM-2 | 200 mg/kg | 3.27±0.14* | 3.42±0.18** | 4.90±0.21** |
4.34±0.20** |
|
AKM-3 |
200 mg/kg | 3.23±0.16* | 3.39±0.17** | 4.85±0.20** |
4.29±0.20** |
Analgesic response is presented as mean ± SEM (standard error of mean), n=6 each group, one-way Analysis of Variance (ANOVA) test was used.
Results and Discussion
Chemistry of Synthesis
All the new acetamide derivatives (AKM-1-AKM-3) were successfully synthesized by a synthetic procedure as shown in Scheme 1. The structures of all the newly prepared compounds were confirmed by infrared spectroscopy, 1H NMR, mass spectroscopy and elemental analysis. First, the 2-chloro-N,N-diphenylacetamide(A) was synthesized by chloroacetylation reaction of diphenylamine with chloroacetyl chloride under the reflux. Cyclization reaction of compound A with thiourea was carried out under the constant stirring with reflux which yield N4,N4-diphenylthiazole-2,4-diamine (B). The compound B on chloroacetylation with chloroacetyl chloride using chloroform as solvent in catalytic amount of potassium carbonate gave 2-chloro-N-(4-(diphenyl amino) thiazol-2-yl) acetamide (C). Further, the compound C on stirring with 4-amino aniline in dry acetone resulted 2-((4-aminophenyl)amino)-N-(4-(diphenylamino)thiazol-2-yl)acetamide (D). The compound 4 was further reacted with different substituted benzaldehydes in GAA in ethyl alcohol, which yielded noval bioactive acetamide derivatives (AKM-1-AKM-3).
Molecular Modeling
The molecular docking study of target compounds (AKM-1-AKM-3) was performed on particular COX enzyme (COX-1 and COX-2) in comparison with a reference drug Diclofenac. The target compounds (AKM-1-AKM-3) demonstrated lower docking scores on COX-1 and COX-2 enzymes, which point out that these, may have better analgesic activity. Among the synthesized compounds, AKM-2 was most potent as it exhibited least level of docking scores for COX-1 and COX-2 receptors.
Pharmacology
Hot plate method was used to screen analgesic action at dose of 200 mg/kg b.w. and Table 3 shows the results of analgesic activity. Diclofenac sodium was employed as a standard drug. Significant analgesic action was exhibited by AKM-2 whereas least analgesic action was showed by AKM-1. The results suggested that the activity response was having similar results as obtained in docking study.
Conclusion
In summary, novel derivatives of 2-chloro-N,N-diphenylacetamide were synthesized by incorporating hydroxy and methyl groups at different position of the aromatic ring and evaluated for analgesic activity through hot plate method. Experimental evidences suggested that compound AKM-2 with one methyl group at the third position in benzaldehyde ring shows significant action. Overall, the compound AKM-2 is a potent analgesic molecule for the treatment of pain by inhibiting COX enzyme, which may be connected with the molecular docking result. The finding of docking study with reference to amino acid interaction residue resulted that there is similarity in interaction residue of Diclofenac (TYR) and AKM-2 (ARG, TYR) for COX-1 receptor. In the same way, similarity was observed in interaction residue for Diclofenac and AKM-2 (TRP) for COX-2 receptor.
Acknowledgement
The authors are thankful to Dr. M.P. Pandey, Honorable Vice Chancellor, IFTM University, Moradabad for the support to complete this work. This work is a part of work done for Ph.D degree of IFTM University, Moradabad.
Conflict of Interest
Nil.
References
- Mousa, T.H.; Rashid, A.M.; Mahdi, M.F. Pharm. Sci. Res., 2019, 11, 531-539.
- Marnett, L.J.; Rowlinson, S.W.; Goodwin, D.C.; Kalgutkar, A.S.; Lanzo, C.A. Biol. Chem., 1999, 274, 22903-22906.
- Janovec, L.; Janočkova, J.; Matejova, M.; Konkoľova, E Bioorganic Chem., 2019, 83, 487–499.
- Nursamsiar; Asnawi, A.; Kartasasmita, R.E.; Ibrahim, S.; Tjahjono, D.H. Appl. Pharm. Sci., 2018, 8, 016-020.
- Guedes, I.A.; Magalhães, C.S.; Dardenne, L.E. Biophysical Rev., 2014, 6, 75-87.
- Rohs, R.; Bloch, I.; Sklenar, H.; Shakked, Z. Acids Res., 2005, 33, 7048-7057.
- Çakir, G.; Küçükgüzel, İ.; Guhamazumder, R.; Tatar, E.; Manvar, D.; Basu, A.; Patel, B.A.; Zia, J.; Talele, T.T.; Kaushik-Basu, N. Pharm. Chem. Life Sci., 2015,38, 10-22.
- Seeliger, D.; Groot, B.L. Comput. Aided Mol. Des., 2010, 24, 417-422.
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cu, M. Comput. Aided Drug Des., 2011, 7, 146–157.
- Jayadevappa, H.P.; Nagendrappa, G.; Umesha, S.; Chandrashekar, S. Adv. Pharm. Tech. Res., 2012, 02, 192–196.
- Ghosh, S.; Tiwari ,P.; Pandey, S.; Misra, A.K.;Chaturvedi, V.; Gaikwad, A.; Bhatnagar, S.; Sinha, S. Med. Chem. Lett., 2008, 18, 4002-4005.
- Ang, W.; Lin, Y.N.; Yang, T.; Yang, J.Z.; Pi, W.Y.; Yang, Y.H. Molecules, 2012, 17, 2248–4458.
- Ozkani, F.; Dalkara, S; Calis, U.; Willke, A. , 1994, 44, 920-924.
- Kumar, S.; Wahi, A.K.; Singh, R. J. Med. Chem., 2011, 46, 4753-4759.
- Kaplancikli, Z.A.;Yurttaş, L.; Turan-Zitouni, G.; Özdemir, A.; Özic, R.; Ulusoylar-Yildirim, Ş. Enz. Inhib. Med. Chem., 2012, 27, 868-674.
- Kumar, A.; Verma, S.; Mishra A.K.; Kumar, S. Annals Adv. Chem., 2017, 1, 053-056.
- Singh, S.; Kumar, V.; Sharma, S.K.; Kumar, A.; Sharma, S. J. Chem., 2010, 26, 93-101.
- Dhall, H.; Kumar, A.; Mishra A.K. Bioact. Comp., 2018, 14, 26-32.
- Eddy, N.B.; Leimbach, D.J. Pharmacol. Exp. Ther., 1953, 107, 38593-38596.
- Kumar, P.; Kumar, A.; Mishra, A.K. Bioact. Comp., 2018, 14, 26-32.

This work is licensed under a Creative Commons Attribution 4.0 International License.









