Acids and Anions Effects on the Distrubution of Cadmium Between Buffered Aqeuous Phases and 4,4´-(1e,1e´)-1,1´-(Ethane-1,2-Diylbis (Azan-1-Yl-1ylidene)) Bis(5-Methyl-2-Phenyl-2,3-Dihydro-1h-Pyrazol-3-Ol) Solutions
J. Godwin1, L. S. Tella1, J. I. Consul2, A. N. Ebelegi1 and N. Ayawei1
1Department of Chemical Sciences, Niger Delta University Wilberforce Island, PMB 71, Bayelsa State, Nigeria.
2Department of Mathematics/Computer Science, Niger Delta University Wilberforce Island, PMB 71, Bayelsa State, Nigeria.
Corresponding Author Email: ayawei4acad@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350611
Article Received on : 19/09/2019
Article Accepted on : 01/11/2019
Article Published : 17 Dec 2019
The effect of some acids, anions and auxiliary complexing agents on the extraction of Cadmium (II) from aqueous solutions buffered to pH 7.5 using a chloroform solution of the Schiff base ligand 4,4´-(1E,1E´)-1,1´-(ethane-1,2-diylbis(azan-1-yl-1ylidene))bis(5-methyl-2-phenyl-2,3-dihydro-1H-pyrazol-3-ol) (H2BuEtP) alone and in the presence of 1-(3-hydroxy-5-methyl-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl) butan-1-one (HBuP) after an equilibration time of sixty minutes was investigated. Working concentration of Cd(II) was 50mgL-1, while a range from 0.001M-3.0M was used for acid and 0.001M-1.0M for anions and auxiliary complexing agents. Extraction raffinates were analysed for Cd(II) using Flame Atomic Absorption spectrophotometry and Distribution Ratios. Percentage Extractions (%E) were calculated by difference of Cd(II) concentrations before and after equilibration. The mixed ligands H2BuEtP/HBuP organic phase was a better extractant for Cadmium than H2BuEtP alone but the difference was not significant for all acids, Cl-, Oxalate and Tartrate. The results indicated that at lower concentrations of the acids, anions and auxiliary complexing agents, a releasing effect occurred with improved extraction of Cadmium > 90% in most cases and at high concentrations there was reduced percentage extraction due to masking of Cadmium from formation of stable salts of Cadmium. Comparing results with other those of other metals studied under same conditions showed that multi-metal extraction with the ligand (H2BuEtP) is possible. H3PO4, H2SO4, HCl, PO43-, EDTA and Oxalate all showed theoretical potentials for separating Cadmium from other studied metals with Separation Factors βxy = Dx/Dy close to and above 104.
KEYWORDS:Cadmium; Acids; Anions; Auxiliary Complexing Agents; 4;4´-(1E;1E´)-1;1´-(ethane-1;2-diylbis(azan-1-yl-1ylidene))bis(5-methyl-2-phenyl-2;3-dihydro-1H-pyrazol-3-ol); Extraction and Theoretical Separation
Download this article as:| Copy the following to cite this article: Godwin J, Tella L. S, Consul J. I, Ebelegi A. N, Ayawei N. Acids and Anions Effects on the Distrubution of Cadmium Between Buffered Aqeuous Phases and 4,4´-(1e,1e´)-1,1´-(Ethane-1,2-Diylbis (Azan-1-Yl-1ylidene)) Bis(5-Methyl-2-Phenyl-2,3-Dihydro-1h-Pyrazol-3-Ol) Solutions. Orient J Chem 2019; 35(6). |
| Copy the following to cite this URL: Godwin J, Tella L. S, Consul J. I, Ebelegi A. N, Ayawei N. Acids and Anions Effects on the Distrubution of Cadmium Between Buffered Aqeuous Phases and 4,4´-(1e,1e´)-1,1´-(Ethane-1,2-Diylbis (Azan-1-Yl-1ylidene)) Bis(5-Methyl-2-Phenyl-2,3-Dihydro-1h-Pyrazol-3-Ol) Solutions. Orient J Chem 2019; 35(6). Available from: https://bit.ly/2M1Qawh |
Introduction
There are numerous literature and ongoing researches on removal of heavy metals from different environments due to reported deleterious health effects associated with these heavy metals [1-3]. One method that has given very encouraging results is solvent – solvent extraction using ligands in appropriate solvent as the organic phases [4-6]. The bases of these extractions is the formation of metal-ligand complexes which are hydrophobic and thus soluble in organic phases, resulting in the distribution of the metal ions from aqueous media to the organic phases [7,8]. These extractions are pH and ligand concentrations dependent and are based on the Nernst distribution law [7]. Assessments in these studies are usually done from distribution ratios (D) and percentage extraction (%E) determinations. The distribution ratio, D, is constant at a particular temperature and is given mathematically as sum of all concentrations of metal ions in organic phase over the concentration of metal ions in aqueous phase. Other factors affecting the distribution of the metal ions between the aqueous and organic phases are: equilibration time, oxidation state of metal ions, presence of a second ligand that can acts as a synergist, presence of acids, anions and auxiliary complexing agents which can act as releasing agents or suppressing/masking agents [7,8,9]. The aim in any metal extraction study is to ascertain conditions in which 99.9% extraction of the studied metal ions can be attained and also note reagents and conditions that can result in suppression/masking of particular metal ions, and thus, can be utilized in the separation of these metal ions from those in which these reagents and conditions enhances extraction of their ions and vice-versa [9,7,10].
Metal extraction studies with Schiff bases (ligands with N=C bonds) show that they have excellent extraction properties which has been attributed to the stability and high hydrophobicity of the metal complexes formed [11-13]. Since its synthesis by Uzoukwu et al., 1998 [14], the Schiff base 4,4´-(1E,1E´)-1,1´-(ethane-1,2-diylbis(azan-1-yl-1ylidene))bis(5-methyl-2-phenyl-2,3-dihydro-1H-pyrazol-3-ol) (H2BuEtP) has been used for extraction studies for Lead [15], Uranium [16], Nickel [17], Iron [18-19] and Cadmium [20]. The effect of acids, anions and auxiliary complexing agents on the distribution of these metal ions between buffered aqueous phases and chloroform solutions of this ligand (H2BuEtP) alone and in the presence of 1-(3-hydroxy-5-methyl-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl) butan-1-one (HBuP) are well reported in these studies [15-19]. These studies showed that common acids (HNO3, HCl, H2SO4, H3PO4 and CH3COOH), anions (NO3–, SO42-, PO43-, CH3COO–, F-, Cl-, Br– and I–) and auxiliary complexing agents (EDTA, Oxalate, Tartarate and SCN–) had varying effects as releasing or masking agents at different concentration and the general trend in most cases was low percent extraction resulting from masking of the metals at higher concentrations of these acids, anions and complexing agents. pH range 4.75 – 7.5 was reported to have > 90% extraction in the study of the distribution of Cd2+ between buffered aqueous media and chloroform solutions of the ligand (H2BuEtP) alone and in the presence of 1-(3-hydroxy-5-methyl-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl) butan-1-one (HBuP) [20]. Slope analysis was used to predict the probable extraction reactions and also the distributing cadmium complexes as Cd(HBuEtP)X (where X is an anion), and Cd(HBuEtP.BuP) respectively. HBuP slightly increased the pH1/2 from 3.87 ± 0.18 to 4.88 ± 0.12 and partition coefficient (KD) from 2.19 ± 0.35 to 3.15 ± 0.42. However, the extraction constant Kex2Cd (-10.09 ± 0.09) in the presence of HBuP was < Kex1Cd (-3.01 ± 0.9) for H2BuEtP alone [20]. However, to completely evaluate the potentials of the ligand 4,4´-(1E,1E´)-1,1´-(ethane-1,2-diylbis(azan-1-yl-1ylidene))bis(5-methyl-2-phenyl-2,3-dihydro-1H-pyrazol-3-ol) (H2BuEtP) for the extraction of Cadmium from buffered aqueous solution, there is a need to determine the effects of common acids, anions and auxiliary complexing agents on the distribution between the two phases. The results from this study indicated that the acids, anions and auxiliary complexing agents used for this study did not significantly enhance the distribution of cadmium from the aqueous phases to the organic phase at pH 7.5. However, when compared with results from previous studies on the distribution of Fe2+, Ni2+, Pb2+ and UO22+ between buffered aqueous media and chloroform solutions of the ligand (H2BuEtP) alone and in the presence of 1-(3-hydroxy-5-methyl-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl) butan-1-one (HBuP), the results indicated theoretical potentials for the utility of the ligand H2BuEtP in the multi-metal extraction of all studied metals and separation of cadmium from lead, iron, nickel and uranium from the varying effects of these acids, anions and auxiliary complexing agents.
Materials and Methods
The ligand (H2BuEtP) was synthesized by methods outlined by Uzoukwu et al., 1998. Elemental analysis for C, H and N; IR and NMR spectral data were done at the Institute for Inorganic Chemistry Technology, University of Dresden, Germany.
Stock solutions of 0.05 M H2BuEtP were prepared by dissolving appropriate amount of the ligand in CHCl3. Stock solutions of 0.05 M HBuP were also prepared by dissolving appropriate amount of the ligand in CHCl3. A 1000 mgl-1 stock solution of Cd2+ was prepared by dissolving 0.256 g of Cadmium Chloride hemi acetate in 100 ml volumetric flask containing 0.2 ml of 10 M HNO3 made up to the 100 ml mark with deionized water.
A pH 7.5 buffer solutions was prepared from 0.1 M NaOH and 0.1 M KH2PO4
The pH of the buffered solutions were determined with a Labtech Digital pH meter. Solutions of 0.001-3.0 M mineral acids or anions/complexing agent concentrations were prepared by diluting appropriate volumes of stock solutions of mineral acid or sodium/ammonium salts of anions/complexing agent. All experiments were performed at ionic strength of 0.1 M (NaClO4).
Two sets of 2mL aqueous solutions of 50mgl-1 Cd (II) containing various concentrations (0.001 – 3.0 M) of acids, or (0.001 – 1.0 M)anions/complexing agents with pH 7.5 were prepared in 10 mL extraction bottles. Two millilitres (2 mL) of the 0.05 M solution of H2BuEtP or 0.05M H2BuEtP:0.05 M HBuP (9:1 ratio by volume) in chloroform were pipetted into the aqueous phases in the extraction containers. The immiscible phases were shaken mechanically for sixty minutes at a temperature of 30 oC. A shaking time of sixty minutes was found suitable enough for equilibration. The two phases were allowed to settle and separated. 0.2 mL of aqueous raffinates were then taken and analysed by difference between the concentration of Cd (II) ions in aqueous phases before and after the extractions using Atomic Absorption Spectrophotometry (AAS) at wavelength of 218nm. Distribution ratio D was calculated as the ratio of metal ion concentration in the organic phase (Co) to that in the aqueous phase (C). Thus D = Co/C.
The two organic phases, ligand H2BuEtP alone and mixed ligands H2BuEtP-HBuP extraction data were statistically analysed using the R software package [21]. The t test statistics [22] was used to test the hypothesis, if the two organic phases were significantly different in these extractions. The null hypothesis (H0), that the two organic phases of interest are not significantly different is rejected if the value of the test statistics is greater than the critical value and the alternative hypothesis(Ha), the two groups of interest are significantly different is accepted. The p value was also used. If the p value is greater than the significant level α = 0.05, the null hypothesis is accepted and we conclude that there is no significant difference between the groups of interest.
Results and Discussion
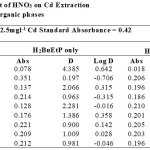 |
Table 1 Click here to View Figure |
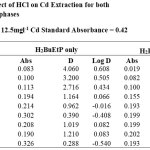 |
Table 2 Click here to View Figure |
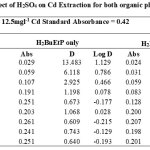 |
Table 3 Click here to View Figure |
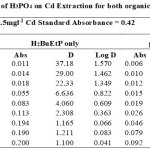 |
Table 4 Click here to View Figure |
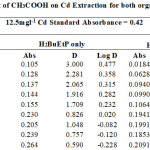 |
Table 5 Click here to View Figure |
 |
Figure 1 Click here to View Figure |
Table 1-17 show the values for the metal standards absorbance and raffinates absorbance from which the extraction parameters; Distribution Ratio D and Percentage Extraction %E were obtained. The plots in Figure 1 showed that the lowest concentration 0.001M of all acids used in the study gave the best percent extraction (75% – 98.5 %) of Cadmium and there was a decrease in almost all cases as the concentration of the acids were increased in both ligand H2BuEtP alone (97.4 – 22.4%) and in the presence of HBuP organic phases (98.5 – 50.2%). The binary ligands H2BuEtP/HBuP organic phase gave slightly better extraction of cadmium with the acids at a concentration of 0.001M when compared with the ligand H2BuEtP alone organic. However, statistically both organic phases extractions in the presence of the acids were not significantly different as p values were all > the significant level of 0.05. Comparing these results with those for Uranium (VI), Pb (II), Ni (II) and Fe (II) in the presence of these acids using same ligand H2BuEtP organic phase are as follow; Uranium (VI) extractions in the presence of the studied acids showed close similarities in percent extractions with Cadmium extractions as 0.001M concentrations of the acids gave highest percentage extractions of metal ions and as the concentration of the acids increased, the percentage extraction of Uranium decreased. However, in the Uranium extraction the binary ligand system gave far higher percentage extractions at this 0.001M acid concentrations that was significantly different from the ligand H2BuEtP alone organic phase as it gave > 90% extraction of Uranium at 0.005M and 0.01M of H3PO4 even in ligand H2BuEtP alone organic phase and 99.91% extraction of Uranium in 0.01M of H3PO4 in binary ligands H2BuEtP/HBuP organic phase [16]. The trend in Iron extraction with studied acids was also similar with results obtained for Cadmium apart from HCl which behaved as salting out agent with increased percent extraction of Iron Fe at > 0.05M of HCl [19]. The results were completely different from those reported for Lead and Nickel extractions with same organic phases in which all acids masks extractions apart from H2SO4 that gave > 64% extraction of Pb and < 10% extraction of Nickel for all concentrations in both organic phases [15,17],
Figure 2 showing percentage extraction of Cadmium in the presence of PO43-, SO42-, NO3– and CH3COO– ion in ligand H2BuEtP alone and in the presence of HBuP. The general trend observed with the acids, where decrease in percentage extraction was observed with increased concentration of the acids. Similar trend was also observed with the anions (93.3% – 11.7% for ligand H2BuEtP alone and 99.1% – 52.9% in the presence of HBuP) with the only notable exception being PO43- in binary ligand system in which highest percent extraction of Cadmium 99.2% was observed at 0.01M PO43-. The Binary ligand H2BuEtP/HBuP organic phase was significantly a better extractant for Cadmium than the ligand H2BuEtP alone system as p values were all < the significant level of 0.05. The trend was similar to those observed for Pb and Ni with same organic phase apart from PO43- in which percent extraction of Pb and Ni increases as concentration of PO43- increases and peaks at 0.1M after which it starts to decrease. This was observed in Lead and Nickel extractions for both organic phases. The trend was also similar for Uranium (VI) but the exception however, was observed in this case with CH3COO– with similar trend as observed for PO43-. The binary ligand system was also significantly a better extractant for Uranium than the ligand H2BuEtP alone organic phase. The trend observed for Cadmium was generally different from those observed for Fe as Iron extraction with same organic phases showed trends observed for PO43- in Pb and Ni and CH3COO– in Uranium in which percentage extraction of metal ions increases with increase in concentrations of the anions [15,16,17,19].
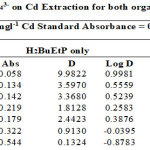 |
Table 6 Click here to View Figure |
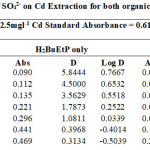 |
Table 7 Click here to View Figure |
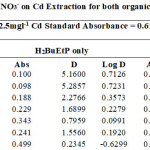 |
Table 8 Click here to View Figure |
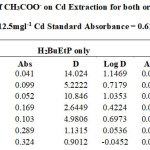 |
Table 9 Click here to View Figure |
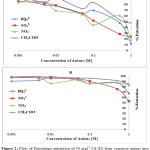 |
Figure 2 Click here to View Figure |
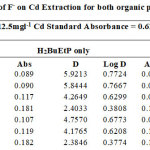 |
Table 10 Click here to View Figure |
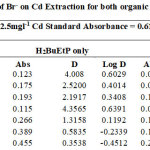 |
Table 11 Click here to View Figure |
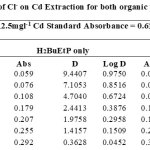 |
Table 12 Click here to View Figure |
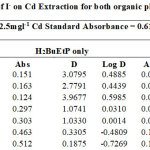 |
Table 13 Click here to View Figure |
The effect of the halogen ions Br–, Cl–, F– and I– in the extraction of Cadmium with H2BuEtP alone and in the presence of HBuP shown in Figure 3 indicate similar behaviour as observed with the acids as there was a steady decrease in extraction of Cadmium Cd as the concentrations of the halogen ions were increased (90.5% – 26.1% for ligand H2BuEtP alone and 98.7% – 66.4% in the presence of HBuP). The binary ligand H2BuEtP/HBuP organic phase was slightly a better extractant for Cadmium than the ligand H2BuEtP alone organic phase as the lowest concentration 0.001 M of the ions used gave > 97% extraction for Cadmium for all halogens ions in binary ligand H2BuEtP/HBuP organic phase as against < 91% for the H2BuEtP alone organic phase. However, statistically the binary ligand H2BuEtP/HBuP organic phase in the presence of Br–, F– and I– was significantly better as an extractant even though the results for Cl– did not give a significant difference. The trend was close to those observed for Lead, Uranium and Nickel even though at 1.0 M of I– complete masking of Lead was observed with the H2BuEtP alone organic phase. The binary ligand H2BuEtP/HBuP organic phase was significantly a better extractant for Uranium and Nickel but only slightly better for Lead. The trend was quite different with those observed for Iron, where the percent extraction of Iron increases and peaks at different concentrations of the halogen ions in the H2BuEtP alone organic phase while in the binary ligand H2BuEtP/HBuP organic phase peaking occurred at 0.01 M for almost all the halogen ions except Br– where peaking occurred at 0.1 M. The H2BuEtP alone organic phase unlike for other studied metal ions studied with this ligand was a slightly a better extractant for Iron Fe than the binary ligand H2BuEtP/HBuP organic phase [15,16,19].
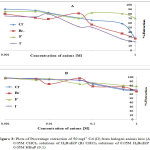 |
Figure 3 Click here to View Figure |
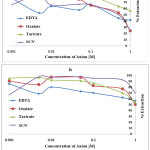 |
Figure 4 Click here to View Figure |
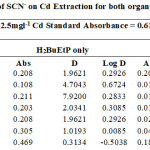 |
Table 14Click here to View Figure |
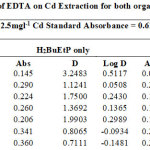 |
Table 15 Click here to View Figure |
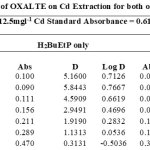 |
Table 16 Click here to View Figure |
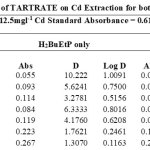 |
Table 17 Click here to View Figure |
The effect of auxiliary complexing agents EDTA, Oxalate, tartrate and thiocyanate (SCN) ions in the extraction of Cadmium Cd with H2BuEtP alone and in the presence of HBuP shown in Figure 4 also indicate a common trend observed with the acids, anions and halogens with the lowest concentration 0.001M giving the highest percent extraction of Cadmium Cd and a steady decrease in percent extraction as the concentration of the auxiliary complexing agents are increased (91.1% – 23.9% with H2BuEtP alone and 95.3% – 50.3% in the presence of HBuP) with a few exceptions. The observed exception with the auxiliary complexing agents was with thiocyanate (SCN) that had 0.005 M as the concentration with the highest percent extraction of Cd in both organic phases (82.5% with H2BuEtP alone and 97.8% in the presence of HBuP) and thereafter, the regular trend of decreasing extraction of Cadmium continued. The binary H2BuEtP/HBuP organic phase was significantly a better extractant than the ligand H2BuEtP organic phase in the presence of SCN– and EDTA but the difference was not significant for Oxalate and Tartrate. With Pb, the highest percent extraction was obtained at different concentrations of the complexing agents and complete masking was observed at 1 M Oxalate and 0.5 M – 1 M Tartrate in ligand H2BuEtP alone organic phase while complete masking at all concentrations was observed for EDTA in both organic phases. The binary H2BuEtP/HBuP organic phase was significantly a better extractant than the ligand H2BuEtP organic phase with HBuP functioning as a synergist with the resultant adducts more hydrophobic than complex with the ligand H2BuEtP alone as reported in related studies [23,24]. Apart from the fact that no masking effect was observed with EDTA in the extraction of Uranium, the results were similar to those observed for Lead. The auxiliary complexing agents did not enhance the extraction of Nickel as results were generally poor and only tartrate gave 70.3% extraction of Nickel at a concentration of 0.1 M in the binary H2BuEtP/HBuP organic phase. Tartrate ion gave > 99% extraction of Iron in all concentrations used for the study in the ligand H2BuEtP alone organic phase. The regular trend of decreasing percent extraction as concentration of complexing agents ions increased as observed for the acids and other anions was also reported. Complete masking of Iron was recorded for EDTA and Oxalate ion from concentrations > 0.05 M [15,16,17,19].
The results obtained with Cadmium in the presence of these acids, anions and auxiliary complexing agents indicate that at some concentration they act as releasing/salting out agents by the formation of unstable salts of Cadmium, thus, making it readily available as free Cadmium ions for complexation with the ligand in the organic phase, resulting in the extraction of the metal from the aqueous phase to the organic phase and in concentrations that the extraction percent is small or zero, they are forming very stable salts with Cadmium and making it unavailable for complexation with the ligand H2BuEtP [25,26]. The results with H3PO4 and PO43- were all very high confirming that Cd3(PO4)2 formed is unstable and salting out of Cadmium is the net effect as reported in related Cadmium extraction study [27,28]. Comparing results with those reported with buffered aqueous solutions of Cadmium (II) at pH 6.0-8.0 which had percent extraction of Cadmium between 99.4%- 99.9% [20] indicated that the acids, anions and complexing agents used for the study did not enhance the extraction of Cadmium at the study pH of 7.5. However, they can be used in the multi-metal extraction of the so far studied metals Lead, Uranium, Nickel, Iron and Cadium with the Ligand H2BuEtP as > 90% extraction was obtained with most of the acids, anions and complexing agents.
Separation Factors βXY
Theoretical Separation Factors βXY were calculated in order to establish the possibility of selectively extracting metals in multimetal media. Table 18- 27 are the tabulated Theoretical Separation Factors βXY for the various metal pairs for ligand H2BuEtP alone and binary ligand H2BuEtP/HBuP organic phases with βXY = Dx/DY close to 104 and theoretical have potential to be used in quantitative separation of Cadmium from Nickel, Iron, Lead and Uranium [29,30].
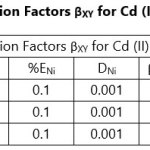 |
Table 18 Click here to View Figure |
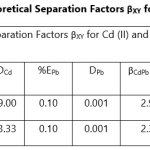 |
Table 19 Click here to View Figure |
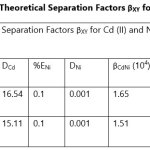 |
Table 20 Click here to View Figure |
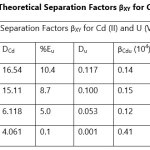 |
Table 21 Click here to View Figure |
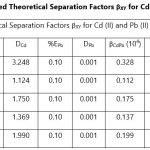 |
Table 22 Click here to View Figure |
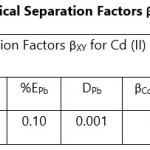 |
Table 23 Click here to View Figure |
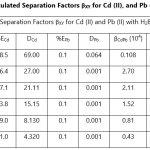 |
Table 24 Click here to View Figure |
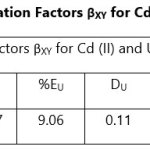 |
Table 25 Click here to View Figure |
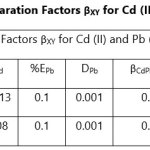 |
Table 26 Click here to View Figure |
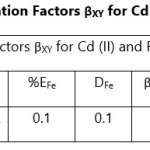 |
Table 27 Click here to View Figure |
The theoretically calculated Separation Factors βXY indicates that the studied acids, anions and complexing agents have great potentials in applications for the separation of cadmium from the so far studied metals from the favourable calculated theoretical separation factors βXY = Dx/DY > 104 and n number of batches needed to achieve 99.9% extraction of a metal using the equation below:
![]() Equation 1
Equation 1
Where C is concentration of metal in aqueous phase after extraction
Caq is initial concentration of metal in aqueous phase before extraction
Since equal volume of aqueous phase and organic phase was used during extractions, equation 1 reduces to;
![]() Equation 2
Equation 2
Table 18 and 20 showed that theoretically, 0.001 M – 0.01 M H3PO4 and 0.001 M H2SO4 with ligand H2BuEtP alone can be exploited for the separation of Cadmium from Nickel requiring 2 batches of extraction and 0.005 M H2SO4 required 3 batches of extraction to obtain 99.9% of Cadmium. Table 19, 22, 23, 24 and 26 showed theoretical conditions for separating Lead with Cadmium. Table 19 showed 0.005 M – 0.01 M H3PO4 for ligand H2BuEtP alone and Table 24 showed 0.001 M – 0.5 M H3PO4 with binary ligands H2BuEtP/HBuP can be used theoretically to separate Cadmium from Lead requiring 2 batches of extraction to obtain 99.9% of Cadmium. Table 22 and Table 23 showed that EDTA and 0.005M HCl can also be used to separate Lead from Cadmium with ligand H2BuEtP alone with 5-8 batches of extraction for EDTA and 5 batches of extraction for HCl with binary ligands H2BuEtP/HBuP theoretically required to obtain 99.9% Cadmium.. Table 21 and 25 show theoretical calculated conditions for separating Cadmium from Uranium with H2SO4 with batches of extractions ranging from 2-5 with increasing concentration with ligand H2BuEtP alone and 0.005 M PO43- requiring 3 batches with binary ligands H2BuEtP/HBuP to obtain 99.9% Cadmium. Table 27 showed that 0.1M Oxalate using the binary ligands H2BuEtP/HBuP will theoretically require 3 batches of extractions to obtain 99.9% of Cadmium
Conclusion
The studied acids, anions and auxiliary complexing agents did not significantly enhance the extraction of cadmium at pH 7.5.
At lower concentrations, most of the acids, anions and auxiliary complexing agents act as releasing agents with > 90% extraction of Cadmium with both ligand H2BuEtP alone and binary ligand H2BuEtP/HBuP organic phase.
Masking effect due to formation of stable salt of Cadmium resulting in lower percent extraction of Cadmium is predominant at higher concentrations of acids, anions and auxiliary complexing agents and more pronounced with ligand H2BuEtP alone organic phase.
The binary ligand H2BuEtP/HBuP organic phase was in all cases slightly a better extractant for Cadmium in the presence of studied acids, anions and auxiliary complexing agents with > 90% extraction for most cases.
The efficiency of the acids in the extraction of Cadmium is the order H3PO4 > H2SO4 > HNO3 > HCl > CH3COOH.
Theoretically, from calculated separation factors βXY, separation of Cadmium from other studied metals can be achieved using; H3PO4 for cadmium from Nickel and Lead , H2SO4 for Cadmium from Nickel and Uranium, HCl and EDTA for Cadmium from Lead, PO43- for Cadmium from Uranium and Oxalate for cadmium from Iron.
Recommendations
Separation of Cadmium from Iron, Nickel and Lead studies based on theoretically favourable conditions be undertaken to ascertain the practical possibility of achieving these separations.
Multi-metal extraction of Cadmium, Iron, Nickel and Lead in the presence of these acids, anions and auxiliary complexing agents be studied for a range of pH using the ligand H2BuEtP alone and in the presence of HBuP.
References
- Khan I. S., Cao Q., Zheng Y. M., Huang Y. Z., and Zhu Y. G. Environmental Pollution. 2008, 152(3), 3686-3692
- Jaishankar M., Tseten T., Anbalagan N., Mathew B. B., and Beeregowda K. N. Interdiscp. Toxicol. 2014, 7(2), 60 -72
- Salazar-Flores J, Torres-Jasso J. H., Rojas-Bravo D., Reyna-Villela Z. M., and Torres Sánchez E. D. J Heavy Met Toxicity Diseases. 2019, 4,1:2, 1-10
- Al Zoubi W. Journal of Coordination Chemistry. 2013, 66(13), 2264-2289
- Soja S. F., Husein H. B., Iwan H., Anna P. and Evamarie H. Procedia Chemistry. 2015, 17, 184-188
- Ekennia A. C., Onwudiwe D. C., Ume C. and Ebenso E. E. Bioinorganic Chemistry and Applications. 2015, Volume 2015, 1-10
- Uzoukwu, B. A. Jenson Service, Rumuomasi Port Harcourt, Millennium Edition. 2009, 166-196
- Al Zoubi W., Kandil F. and Chebani N. K. Arabian Journal of Chemistry. 2016, 9(4), 526-531
- Thomas J., G., Surender G. D. and Reddy M. L. P. Separation Science and Technology. 2003, 38:15, 3761-3774
- Gergoric M., Christian Ekberg C., Britt-Marie Steenari B. and Retegan T. Journal of Sustainable Metallurgy. 2017, 3(3), 601–610
- Xiao-yuan, C., Shu-zhong, Z., and Qing-jin, M. Transition Met. Chem. 1996, 21, 345
- Epstein D. M., Choudhary S., Churchill M. R., Keil K. M., Eliseev A. V., and Morrow J. R . Inorg. Chem., 2001, 40 (7), 1591–1596
- Zhi-Qiang F., Xiao-Li Y. and Yuan-Feng Ye. ScientificWorld Journal. 2013, 1-9
- Uzoukwu, B. A., Gloe, K., and Duddeck, H. Indian Journal of Chemistry. 1998, 37B,1180 – 1183
- Godwin, J., and Uzoukwu, B. A. Journal of Applied Chemistry (IOSRJAC). 2012a, 1(3),14-21
- Godwin J., and Uzoukwu, B. A. International Journal of Chemistry. 2012b, 4(4), 105-116
- Godwin, J., Nwadire, C. F., and Uzoukwu, B. A. Eur. Chem. Bull. 2012, 1(7), 269-273
- Godwin, J., Chukwu U. J., and Gad T. D. Journal of Advances in Chemistry. 2013, 8(2), 1581-1589
- Godwin, J., Inengite A. K., and Chukwu U. J. International Journal of Chemical and Process Engineering Research. 2014, 1(7), 59-72
- Godwin, J., and Tella, L. S. Anachem Journal. 2017, 7(1), 1329-1340
- R. Development Core Team. 2008, Vienna, Austria.
- Sprinthall R. C. Allyn and Bacon Inc, Boston, United States. 2011, 183-213.
- Petrova M. A. and Dukov I. L. Journal of Flow Chemistry. 2008, 62(2), 207-214
- Jawad S. K. and Husien N. S. M. International journal of Engineering and Technology. 2018, 7(4,36), 553-556
- John J. S. and Du Preez A. C. Solvent Extraction and Ion Exchange. 2002, 20(3), 359-374
- Guezzen B., and Didi M. A. American Journal of Analytical Chemistry. 2015, 6(11), 898-910
- Skorovarov J. I. J. Radioanal Nuc. Chem. 1998, 229, 1-2, 111-116
- Senhaji S. B., Elyahyaoui A., Boulassa S., and Essassi E. M. Orient J. Chem. 2016, 32(6)
- Devi N. Elsevier Transactions of Nonferrous Metals Society of China. 2016, 26(3), 874-881
- Kumari A., Sahu K. K. and Sahu S. K. Metals. 2019, 9, 269

This work is licensed under a Creative Commons Attribution 4.0 International License.









