Electronic Structure and Spectral Study of Some Five Coordinate Complexes of Copper (II)
Birendra Kumar*1 , Rekha Rani2, Dayanand Prasad2, Praveen Kumar Singh1, Amit Kumar2 and Shivadhar Sharma2
, Rekha Rani2, Dayanand Prasad2, Praveen Kumar Singh1, Amit Kumar2 and Shivadhar Sharma2
1Department of Chemistry, Jagjiwan College, Gaya.
2Department of Chemistry, M. U. Bodh-Gaya.
Corresponding Author E-mail: birendra5556@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350430
Article Received on : 06-06-2019
Article Accepted on : 16-08-2019
1-phenylazo-2-nephthol has been synthesized and used for complexation with Cu(II) metal ion along with pyridine, α-picoline, β- picoline, γ-picoline and water as secondary ligands. On the basis of elemental analysis and molar conductivity complexes were formulated as CuL2X [Where L is the prime ligand i.e 1-phenyl-azo-2-nephthol and X is the secondary ligand i.e. pyridine, α-picoline, β-picoline, and H2O.] The magnetic moment of these complexes (1.80 - 1.83 BM) indicates that these complexes are magnetically dilute. The appearance of 3 bands in the electronic spectra of complexes rules out the trigonal bipyramidal (D3h) symmetry arround Cu(II) ion in these complexes rather the electronic spectra favours square pyramidal (C4V) symmetry of these five coordinate complexes. The highest value of 10Dq clearly indicates the greater coordinating ability of α-picoline than pyridine, β-picoline and γ-picoline.
KEYWORDS:α-picoline, β- picoline, γ-picoline, trigonal bipyramidal [D3h], square pyramidal [C4V], five coordinate complexes, magnetically dilute
Download this article as:| Copy the following to cite this article: Kumar B, Rani R, Prasad D, Singh P. K, Kumar A, Sharma S.Electronic Structure and Spectral Study of Some Five Coordinate Complexes of Copper (II). Orient J Chem 2019;35(4). |
| Copy the following to cite this URL: Kumar B, Rani R, Prasad D, Singh P. K, Kumar A, Sharma S.Electronic Structure and Spectral Study of Some Five Coordinate Complexes of Copper (II). Orient J Chem 2019;35(4). Available from: http://www.orientjchem.org/?p=59287 |
Introduction
The well known story of blue proteins which have been extensively explored due to their involvement in oxygen and electron transfer in physiological reactions, is well documented 1-2. Metal ions are electron deficient while most of biological molecules like proteins, DNA , etc. are of these opposing charges leads to a general tendency to form metal ions polymers 3-4 . Chemical nucleaes based on the transition metal ions, cleave DNA hydrolytically or oxydatively with or without added reductant. Cu(II) hypyrimol complex [Cu(Hypyrimol)Cl] has been reported to cleave φX174 supercoiled DNA efficiently without any reductant and also showed high cytotoxicity toward L1210 murine Leukemia and A2780 human, ovarian carcinoma cancer cell lines5-6. Riboflavin binding protein responsible for active transport and storage of riboflavin in egg needed for developments also binds Cu7. Dinuclear complexes of Cu are also well known artificial metalonucleases and metaloprotenase8.Recently superoxide dismutase and antimicrobial activities of emidazolate bridged dinuclear complexes of Cu(II) have also been reported9.
Recently D.Day et al10 have reported five coordinate Cu(II) complexes with a N, O donor Schiff-base ligand which showed their effective participation in CT-DNA binding and pBR322 DNA cleavage activities.
The interest in five coordinate complexes took root in 1974 when M.Nonoyama11 studied five coordinate Ni(II) and Cu(II) complexes and reported their magnetic moments, infrared and electronic spectra.
Albertin et al12 have reported the synthesis and properties of five coordinate Cu(II) complexes with amine ligand.Milan Melnik et al13 have made a review in 2006 on five coordinate Cu(II) complexes.The review summarises the data over a good number of four and five coordinate Cu(II) complexes, mostly with square pyramidal symmetry. The interesting structural features of five coordinate complexes with either square pyramidal or trigonal bipyramidal symmetry invited our attention to five coordinate complexes. In continuation of our previous work 14-17, in the present paper we report the synthesis and spectral properties of some five coordinate Cu(II) complexes with 1-phenylazo-2-nephthol (PANH) as prime ligand and α-picoline, β- picoline, γ-picoline and water as secondary ligand.
Material and methods
All the reagents used were of AnalR grade and were used without further purification. Benzene diazonium chloride and 2-nephthol were procured from Marc.India and copper chloride hexa hydrate (CuCl2.6H2O) was purchased from Nice. The ligand 1-phenylazo-2-nephthol (PANH) was prepared by heating the mixture of 0.01 mole (1.425 gms) of benzene diazonium chloride and 0.01 mole (1.44 gms) of 2-nephthol in ethanolic solution for one hour with stirring. On cooling the solution at room temperature, a brown pericipitate appeared which was filtered and washed with alcohol several times. It was recrystalized in alcohol and dried in an electric oven at 900 C. The yield was found about 80% and its melting point was recorded 1150 C. The ligand was used for complaxation with Cu(II) by the usual method of reflux of the ethanolic solution of 0.01 mole of CuCl26H2O, 0.02 mole of PANH and 0.01 of the secondary ligands pyridine or α-picoline or β- picoline or γ-picoline. Pyridine, α-picoline, β- picoline and γ- picoline were used as secondary ligand. Complexes were recrytallized in 1:1 ethanol-acetone solvent. The micro analysis of carbon, hydrogen and nitrogen in ligand as well as complexes were carried out by using micro analysis technique on Carbo Erba Micro Analyzer 1108. Copper content in complexes was determined iodometrically. The molar conductivity of the complexes was measured at room temperature with its DMSO solution of 10-3 M concentration using Toshniwal digital conductivity meter with deep type cell. The I.R. Spectra of the ligand, 1-phenylazo-2-nephthol and its Cu(II) complexes were recorded on Perkin – Elomer Model Arc RX1 Spectrophotometer. The magnetic susceptibility of the complexes was measured at room temperature by Gouy’s balance using CuSO4.5H2O as calibrant. The electronic spectra of complexes were recorded on Perkin-Elmer Lambda 950 spectrophotometer. The analytical data, magnetic moment and molar conductivity have been presented in table 1.
Table 1: Percentage Composition (%) Found/Calculated, Molar Conductivity and Magnetic Moment
|
Compounds |
Colour | M.P. | Metal | C | H | N | λm(ohm-1 cm2mol-1) |
µ (BM) |
|
|
1. |
1-phenylazo-2-naphthol(PANH) | Brown | 1150C | — | 77.64/77.42 | 4.53/4.84 | 11.10/11.29 | — |
— |
|
2. |
[Cu(PAN)2(H2O)] | Green | 2590C | 10.96/11.03 | 66.82/66.72 | 4.00/4.14 | 9.61/9.73 | 24 |
2.00 |
|
3. |
[Cu(PAN)2(Py)] | Light Green | 2650C | 9.81/9.98 | 69.91/69.76 | 4.13/4.24 | 10.84/11.00 | 20 |
1.80 |
|
4. |
[Cu(PAN)2(α-Pico)] | Light Green | 2780C | 9.66/9.76 | 70.30/70.10 | 4.28/4.46 | 10.62/10.76 | 22 |
1.83 |
|
5. |
[Cu(PAN)2(β-Pico)] | Dull Green | 2580C | 9.64/9.76 | 70.35/70.10 | 4.26/4.46 | 10.66/10.76 | 20 |
1.82 |
|
6. |
[Cu(PAN)2(ϒ-Pico)] | Bright Green | 2590C | 9.62/9.76 | 69.32/70.10 | 4.32/4.46 | 10.58/10.76 | 18 |
1.82 |
Result and Discussion
The molar conductivity of complexes falls in the range of 18-24 ohm-1 cm2mol-1, which is indicative of non electrolytic nature of complexes 18-22. On the basis of elemental analytical data and molar conductivity the complexes have been formulated as [Cu(L2)X] where L is 1-phenylazo-2-nephthol (PANH) and X is H2O , pyridine, α-picoline, β-picoline and γ-picoline. Out of cumbersome infrared spectra of the free ligand and complexes, some important bands of interest have been discussed here. The IR spectrum of the free ligand displays a broad band at 3200 cm-1 which may be assigned to intramolecular hydrogen bonded phenolic group vibration.23-25 The possibility of intramolecular H-Bond formation in the ligand may be seen from its structure.
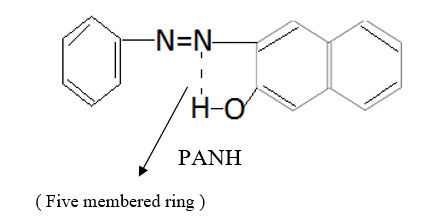
This band disappears in the spectrum of all the complexes of Cu(II) indicating the deprotonation of phenolic O-H group and subsequent coordination through it26-28 . This supposition is further confirmed by red shift in the absorption frequency of νAr-C-O stretching vibration, from 1040 cm-1 in the spectra of free ligand to 1050-1055 cm-1 in the spectra of complexes29-31. The bands appearing at 1610 cm-1 (weak and sharp), 1525 cm-1 (strong and sharp) and 1480 cm-1 (medium and sharp) may be assigned to aromatic ring vibration32-33. The weak and broad band appearing at 1455 cm-1 in the spectra of free ligands as well as complexes is assigned to the ring vibration of two condensed ring system i.e. naphthalene34-35. Thus the presence of both benzene and naphthalene rings in the ligand and complexes is confirmed. A very weak band appearing at 1410 cm-1 in the IR spectra of free ligand has reasonably been assigned to ν-N=N- stretching vibration36. This band is found to have undergone blue shifts in the spectra of complexes wherein it absorbs at 1380-1385 cm-1. This negative shifting in stretching vibration of –N=N- (azo) group is indicative of its involvement in coordination to Cu (II) in complexes37-38. This coordination may further be substantiated by an increase in absorption frequency of ν-C-N stretching which shifts from 1290 cm-1 in the IR spectrum of free ligand to 1295-1300 cm-1 in the spectra of all the complexes. A tacit support from such proposition may be brought out more clearly from far infrared range wherein two new vibration bands appear in the range of 490-510 cm-1 due to ν-Cu-N and at 410-420 cm-1 due to ν-Cu-O 39-41. In addition to these bands some new bands also appear in spectra of complexes.A broad band appearing at 1400 cm-1 with another band at 910 cm-1 are the diagnostic band for coordinated water42-43. The appearance of new bands at 755-795 cm-1 is the signature of the presence of coordinated pyridine, α-picoline, β-picoline and γ-picoline in complexes number 3,4,5 and 6 respectively.44-45. The graphs of FTIR spectra of the ligand as well as its complexes have been given in fig 1-5.
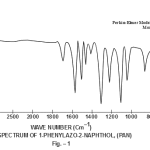 |
Figure 1 Click here to view figure |
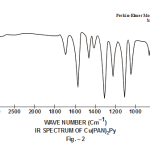 |
Figure 2 Click here to view figure |
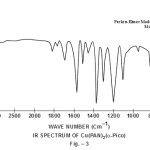 |
Figure 3 Click here to view figure |
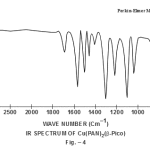 |
Figure 4 Click here to view figure |
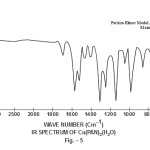 |
Figure 5 Click here to view figure |
The magnetic moment values of complexes fall in the range of 1.80-2.00BM, which is greater than µs for 1-unpaired electron in d9 System i.e., 1.732BM.It shows that all the complexes are magnetically dilute. The values however couldn’t predict the geometry of complexes46-48.
From the stoichiometric formula derived from elemental analysis and molar conductivity it may be inferred that Cu(II) complexes are five coordinated, which may either be of trigonal bipyramidal symmetry (D3h) or square pyramidal geometry (C4v).49 It has been reported that though a trigonal bipyramidal structure is eventually favoured for a five coordinate species, the energy difference between the two is in general rather small. So square pyramidal symmetry for five coordinate complexes may actually be favoured especially when ligands are large and bulki50-51.
The other major point to predict the symmetry of a five coordinate complex is that for a complex of D3h symmetry the electronic spectral bands at 10,500-14,600 cm-1 are observed with greater absorption intensity to the lower energy.52-53 The five coordinate complexes of C4v symmetry display electronic spectral bands in the range of 11400-15000 cm-1 with greater absorption intensity of the band of higher energy. Secondly the splitting pattern of 2D-term of d9 system. Under the perturbation of D3h crystal field and C4v crystal field may be given as below:-
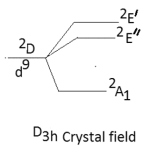 |
Scheme 1 Click here to view Scheme |
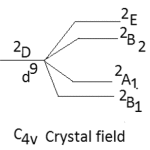 |
Scheme 2 Click here to view Scheme |
Thus in the Cu(II) complexes of D3h system only two bands are expected while for complexes of C4v symmetry, tree bands are expected in their electronic spectra. Here in our study the five coordinate Cu(II) complexes display three bands in their electronic spectra which are given below in table-2 with their assignment.
Table 2: Electronic spectral band in cm-1
| Compounds | ν1 | ν2 |
ν1 |
|
|
1. |
[Cu(PAN)2(H2O)] | 11,580 | 14,250 |
14,700 |
|
2. |
[Cu(PAN)2(Py)] | 11,500 | 14,000 |
14,800 |
|
3. |
[Cu(PAN)2(α-Pico)] | 11,550 | 14,150 |
14,900 |
|
4. |
[Cu(PAN)2(β-Pico)] | 11,600 | 14,050 |
15,000 |
|
5. |
[Cu(PAN)2(γ-Pico)] | 11,540 | 14,020 |
14,940 |
Assignment of these bands may be given as below.
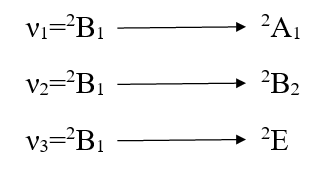
Since the ν2 is the measure of 10Dq. So 10Dq values of these complexes [Cu(PAN)2(H2O)], [Cu(PAN)2(Py)], [Cu(PAN)2(α-Pico)], [Cu(PAN)2(β-Pico)] and [Cu(PAN)2(γ-Pico)] are 14,250, 14,000, 14,150, 14,050 and 14,020 CM-1 respectively. Since the planner ligand PANH is the same producing the same crystal field the change in Dq value has been caused by the comparable strength of the ligand along Z-axis. The values are in good arrangement with the values reported for Cu(II) complexes of square pyramidal geometry54-56.
Conclusion
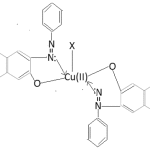
On the basis of the forgoing study of electronic spectra of complexes whole the Cu(ii) complexes have been found to posses square pyramidal (C4v) symmetry. The tentative structure is given as below.
 |
Scheme 3 Click here to view Scheme |
Cu(PAN)2X
X=H2O, Py, α-picoline, β-picoline, γ-picoline
Acknowledgement
The authors are thankful to Dr. R.P.S. Chauhan, Professor and Head. Department of Chemistry, to, allowing us the labs at University department of Chemistry and also directing Technician Dr. Md. Alamgir to record the FTIR and Electronic spectra of complexes.
References
- Uhlmann, E.U.; Peymann, A. Rev. 1990,90,543.
- Sigman, D.S.; Mazumdar, A.; Perkin, D.M. Rev. 1993,93,2295.
- Schreiber, S.L.; Bioorg. Chem. 1998, 6, 1127.
- Jemieson, E.R.; Lippard, S. J. Rev. 1999, 99, 2467.
- Maheshwari, P. U.; Ray, S.; Dulk, H.; den Barends, S.; Wezel, G.; Van Kozlevcar, B.; Gamez, P.; Reedijik, J. Am. Chem. Soc. 2006,128,710.
- Maheshwari, P. U.; Der Ster, M.V.; Smulders, S.; Brends, S.; Wezel, G.; Van. Massera, C.; Roy, S.; Dulk, H. Den.; Gamez, P.; Reedijk, J. Inorg. Chem. 2008,74,3719.
- Smith, S. R.; Pala, I.; Parsons, M.B. Inorg. Biochem. 2006,100,1730.
- Liu, C.; Wang, M.; Zhang, T.; Sun, H. Chem. Rev. 2004, 248, 147.
- Bharti, S.; Chaudhary, M.; Rawat, S. P.; Sangeeta, Noorussabah, Ahmad, K.; Sharma, S. R.; Saket, S.S.; Singh, B.; Das, B.; Kumar, S. Indian. Chem. Soc. 2016, 93, 953.
- Day, D.; Pal, S.; Bag, P. P.; Saha, S.; Chandraleka S.; Dhamsekran, D.; Kole, N.; Biswas, B. Ind. Chem. Soc. 2015, 92, 191.
- Nonoyama, M. Chimi. Acta, 1975, 10, 59.
- Albertin, G.; Bordignon, E.; Orio, A. A. Am. Chem. Soc. 1975, 14, 1411.
- Melnik, M.; Kavesova, M. Dunaj-Jurco, M.; Clive, E.; Holloway, Coord. Chem. 2006, 35,182.
- Kumar, B.; Kumar, R.; and Kumar, B. J. Chem. 2015, 31(3), 1827.
- Kumar, B.; Kumar, B. Kumar, S.; Kumar, D.; and Sharma, S. Orient J. Chem. 2017, 33(5), 2643.
- Kumari, B.; Kumar, B.; Kumar, S.; Kumar, D.; Sharma, S. J. Engg. Sci. Inv. 2018, 7(5), 14.
- Kumar, V.; Singh, R. K.; Kumari, V.; Kumar, B.; Sharma, S. J. Chem. 2018, 34(4), 1937.
- Kumari, P.; Prakash, S.; Prakash,, D. Ind. Chem. Soc. 2012, 89, 19.
- Rama, I.; Rama Shwami, S. Ind. Chem. Soc. 2014, 91, 1877.
- Kumar, B.; Kumar, B. Kumar, S.; Kumar, D.; and Sharma, S. Orient J. Chem. 2017, 33(5), 2643.
- Kumari, B.; Kumar, B.; Kumar, S.; Kumar, D.; Sharma, S. J. Engg. Sci. Inv. 2018, 7(5), 14.
- Kumar, V.; Singh, R. K.; Kumari, V.; Kumar, B.; Sharma, S. J. Chem. 2018, 34(4), 1937.
- Rahman, F.; Hiremath, B.; Basava Rajaiah, S. M.; Jaya Kumar Oswamy, B. H. M.; Swami, M. Ind. Chem. Soc. 2008, 85, 381.
- Mahapatra, B. B.; Sarangi, A. K. Ind. Chem. Soc. 2009, 86, 559.
- Kumar, D.; Shayamal, A.; Kumar, A.; Gupta, P. K.; Das, D. Ind. Chem. Soc. 2010, 87, 417.
- Mishra, L. K.; Keshri, B. K. J. Chem. Sect A. 1981, 28, 863.
- Singh N. K. and Singh S. B., J. Chem. Sect A. 1993, 40, 1070.
- Raman, M.S.; Maninaram, A.; Arunachalan, S.; Krishnan ,C. J.; Chinnuswami, V. Ind. Chem. Soc., 2008, 85, 988.
- Shriniwashan, R.; Verghese.;B.; Sougand, I.; Velaban, K.; Vendkatesan, R.; Rao, P. S. 2004, 23, 115.
- Prabhakaran, R.; Krishnan, V.; Karbendu, R.; Geetha, A.; Vertognolli.; Natrajan. K. Chem. Acta. 2006, 359, 1114.
- Das, A. K.; Dev, D.; Bhowmik, K. R. N.; Purkayastha, R. N. D. Ind. Chem. Soc. 2009, 86, 76.
- Thangadurai, T. D.; Gowri, M.; Natrajan, K. React. Inorg. Metal. Org. Chem. 2002, 32, 329.
- Singh, M. K.,Laskar, A. D. R.; Paul, B. Ind. Chem. Soc. 2008, 85, 485.
- Nakanishi, K.; “Infrared absorption spectroscopy”, Holden Day, Inc. Sanfrancisco and Nankodo company Ltd. Tokyo, Second Printing, 1964, 30.
- Chandra, S.; Jain, D.; Sarkar, A.; Anupama. Ind. Chem. Soc. 2009, 220.
- Williams, D. H.; Flemming, I. “Spectroscopic method inorganic Chemistry”, TATA Mc Graw Hill Publishing company Ltd. New Delhi, 4th Eighth reprint, 2001, 52-53.
- Kumaran Nair, M.L.H.; Shyamla, L. Ind. Chem. Soc. 2009, 86, 133.
- Balasubramanian, K. P.; Raju, V. V.; Chinnusamy, V. Ind. Chem. Soc. 2009, 86, 570.
- Ahmad, I.; Akhtar, F. J. Chem. 1981, 20A, 737.
- Raman, M. Ind. Chem. Soc. 2008, 85, 1082.
- Krishnankutty, K.; Ummathur, M. D.; Saynder, P. Ind. Chem. Soc. 2009, 86, 325.
- Nakamoto, K. “Infrared spectra of Inorganic and coordination Compounds” , John Wiley and Sons, Inc. New York, 1953,P- 156.
- Saha, N.; Dutta, K. N.; Adak, A. K. J. Chem. 1981, 20A, 744.
- Albrecht, M. G.; Creghton, J. A. Amer. Chem. Soc. 1977, 99, 5215.
- Yamada, H. Spectros. Rev. 1981, 17, 227.
- Sunatsuki,Y.; Motoda,Y.; Matsumoto,N. Chem. Rev. 2002, 226, 199.
- Roth, A.; Becher, J.; Hermann, C.; Gorla, H.; Vaughan, G.; Ritcher, M.; Klemn, D.; Plass, W. Chem. 2006, 45, 10066.
- Mishra, A. P.; Kumar, K. Ind. Chem. Soc. 2009, 86, 1150.
- Mutterties,; Schunn. Rev. (London). 1966, 20, 245.
- Brewster, J. A.; Sarage, C. A.; Venanze. Chem. Soc. 1961, 3699.
- Hertley, J. G.; Verangie, L. M.; Goodall, D. C. Chem. Soc. 1963, 4930.
- Nakamoto, K.; Mc Carthy, P. J., “Spectrocopy and structure of metal chelate compounds”, John Wiley and Sons Inc. New York, 1968,P- 117-122.
- Bencini, A.; Gatteschi, D. Chem. 1977, 16, 1994.
- Hathaway, B. J.; Billing. Coord. Chem. Rev. 1970, 5, 143.
- Hitchman, M. A. Chem. 1974, 13, 2218.
- Kumar, B.; Sangal, S. K.; Kumar, A. Ind. Chem. Soc. 2009, 86, 1038.

This work is licensed under a Creative Commons Attribution 4.0 International License.









