Synthesis of Some New Azo Compounds of Salicylic Acid Derivatives and Determine Their In Vitro Anti-Inflammatory Activity
Husam Hamza Salman*1 , Huda Salih Abood1 and Usama Hamid Ramadhan2
, Huda Salih Abood1 and Usama Hamid Ramadhan2
1Department of Pharmaceutical Chemistry, College of Pharmacy, University of Basrah, Iraq.
2Department of Laboratories Clinical Science, College of Pharmacy, University of Basrah, Iraq.
Corresponding Author E-mail: hussam_712003@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/350251
Article Received on : 14-01-2019
Article Accepted on : 07-04-2019
Article Published : 29 Apr 2019
This study included the preparation of a series of some new azo compounds by diazo coupling aromatic amines with salicylic acid derivatives. The prepared compounds identified using precise elemental analysis (C.H.N.), the results supported the structure of concerned compounds. The synthesized azo compounds also identified by using infrared spectroscopy and 1H-NMR spectroscopy. Anti-inflammatory activity of the compounds were determined in-vitro by human red blood cell (HRBC) membrane stability method, the compounds showed a significant activity to protection of the cell membrane. Other compounds show moderate to low activity, sodium diclofenac was used as positive control.
KEYWORDS:Azo Compounds; In-Vitro Anti-Inflammatory and HRBC; Salicylic Acid
Download this article as:| Copy the following to cite this article: Salman H. H, Abood H. S, Ramadhan U. M. Synthesis of Some New Azo Compounds of Salicylic Acid Derivatives and Determine Their In Vitro Anti-Inflammatory Activity. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Salman H. H, Abood H. S, Ramadhan U. M. Synthesis of Some New Azo Compounds of Salicylic Acid Derivatives and Determine Their In Vitro Anti-Inflammatory Activity. Orient J Chem 2019;35(2). Available from: https://bit.ly/2UNvgTj |
Introduction
Compounds containing in their structure a group or more of the AZO groups (–N=N–) called azo compounds,1 in which the nitrogen atom hybridization is sp3.2 Because of their physical and chemical properties as well as their biological efficacy, they possess several important applications in pharmaceuticals, cosmetics, textile industry, analytical chemistry and food.1
Azo compounds are well known to have medical importance, they are recognized in many applications such as antidiabetics,1 and they involved in many biological reactions such as inhibition RNA, DNA, protein synthesis carcinogenesis, and nitrogen fixation.3 Azo compounds are studied as HIV inhibitors of viral replications.4 Because of the presence of the azo moiety make these compounds possess biological efficacy as anti-bacterial,5 antitumor6 insecticide and pesticidal7,8 activities. Salicylate compounds are widely valued because of their antipyretic, pain killing and anti-inflammation properties. 2-Hydroxybenzoic acid (called also salicylic acid) is most commonly used, and known in salicylates class compounds.9
The aim of study synthesis of some new azo compounds of salicylic acid derivatives by the diazo coupling reaction and determine their in-vitro anti-inflammatory activity by human red blood cell (HRBC) membrane stability method.
Experimental
Material and Methods
p-Nitroaniline, p-aminobenzoic acid, m-nitroaniline, 3-methoxysalicylic acid, 3-methylsalicylic acid and 4-methylsalicylic acid and solvents were used in this study sourced from Sigma-Aldrich company and Merck Company. The purity of prepared compounds was checked by thin layer chromatography. Melting points recorded by using Gallenkamp apparatus. FT-IR spectra (KBr) of prepared compounds determined on Shimadzu spectrometer (400-4000 cm-1). The proton nuclear magnetic resonance (1H-NMR) spectra determined on Bruker-NMR spectrometer at 300 MHz using an internal standard Me4Si (TMS) and deuterated DMSO-d6 as a solvent. Elemental analysis and 1H-NMR spectra carried out in Al-albayt University Amman / Jordan.
General method for synthesis of diazonium salts
A solution of an aromatic amine (5 mmol), 1.5 ml of water and 1.5 ml concentrated HCl kept cooled in an ice-salt bath (0°C). A solution of sodium nitrite (5.5 mmol) in 1.5 ml of water added slowly with stirring. The mixture kept at 0°C. for the next step.10,11 The other diazonium salts synthesized in a similar procedure. Figure (1) show synthesis of diazonium salt.
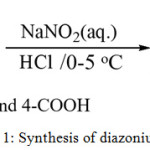 |
Figure 1: Synthesis of diazonium salt. |
General Method for Synthesis of Azo Compounds
The prepared solution of diazonium salt was added portion wise to a solution prepared from salicylic acid derivatives (5.4 mmol) and 10 ml of 2.5 M aq. Sodium hydroxide. The mixture kept with stirring at (0-5oC) for 3-5 hr. The mixture then acidified with conc. HCl (1.5 ml) up to pH ≈ 3. The precipitated compound separated and washed with H2O. The desired product dried and recrystallized with glacial acetic acid.10,11 Figure (2) show synthesis of azo compounds. The melting points, names and the percentage of yield are given in Table (1).
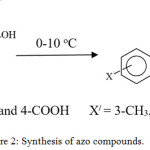 |
Figure 2: Synthesis of azo compounds. |
Table 1: Dames and physical properties of synthesized azo compounds.
|
Comp. |
X |
X/ |
Name |
M. p. (°C) |
Appearance |
Yield (%) |
|
A |
3-CH3 |
4-NO2 |
2-Hydroxy-3-methyl-5-(4-nitro-phenylazo)-benzoic acid |
273-275 dec. |
Yellow |
77 |
|
B |
3-CH3 |
3-NO2 |
2-Hydroxy-3-methyl-5-(3-nitro-phenylazo)-benzoic acid |
243-245 |
Light yellow |
70 |
|
C |
3-CH3 |
4-COOH |
5-(4-Carboxy-phenylazo)-2-hydroxy-3-methyl-benzoic acid |
265-267 dec. |
Red |
83 |
|
D |
3-OCH3 |
4-NO2 |
2-Hydroxy-3-methoxy-5-(4-nitro-phenylazo)-benzoic acid |
245-247 dec. |
Deep red |
78 |
|
E |
3-OCH3 |
3-NO2 |
2-Hydroxy-3-methoxy-5-(3-nitro-phenylazo)-benzoic acid |
236-238 |
Yellow |
89 |
|
F |
3-OCH3 |
4-COOH |
5-(4-Carboxy-phenylazo)-2-hydroxy-3-methoxy-benzoic acid |
253-255 dec. |
Brown |
80 |
|
G |
4-CH3 |
4-NO2 |
2-Hydroxy-4-methyl-5-(4-nitro-phenylazo)-benzoic acid |
235.238 dec. |
Orange |
91 |
|
H |
4-CH3 |
3-NO2 |
2-Hydroxy-4-methyl-5-(3-nitro-phenylazo)-benzoic acid |
240-242 dec. |
Deep yellow |
79 |
|
I |
4-CH3 |
4-COOH |
5-(4-Carboxy-phenylazo)-2-hydroxy-4-methyl-benzoic acid |
248-250 dec. |
Deep red |
88 |
In Vitro Anti-Inflammatory Activity12
HRBC method was used to estimation in vitro anti-inflammatory activity. Blood was collected from healthy volunteers; the blood was mixed with equal volume of sterilized Alsever’s solution. The blood solution centrifuged at 3000 rpm and the packed cells were separate. The packed cells were washed with isosaline solution and 10% v/v suspension was prepared by complete the volume with isosaline. Alsever’s solution were prepared of 2.05% glucose, 0.42% NaCl, 0.8% trisodium citrate, 0.055% citric acid, all dissolved in water. This solution was using for storage RBC. Other solution were using in this method Hyposaline (0.7% NaCl), Isosaline (0.9% NaCl), phosphate buffer (pH 7.4) and ethanol.
Tested compounds (75 mg) was dissolve in 1ml of ethanol. Samples of each compound, control and sodium diclofenac were separately mixed with (1ml) phosphate buffer, (2ml) of hyposaline and (0.5ml ) of HRBC suspension. All the assay mixtures were incubate at 36.5ºC for 30 minutes and centrifuged at 3000 rpm for 10 minutes. The supernatant liquid was decanted and haemoglobin content was estimate by spectrophotometer at 560 nm. The percentage of haemolysis protection was estimate by assuming the haemolysis produced in the control as 100%, according to following equation.
Percentage protection=100-(Ac-As/Ac)
Were Ac= Absorption of control and As= Absorption of sample
Statistical Analysis
The results of anti-inflammatory activity were analysis in one way analysis of variance (ANOVA). Value with probability (p<0.01) was considered significant.
Results and Discussion
Table (2) show CHN analysis of synthesized azo compounds (A-I) and the practical results support the structure of synthesized compounds.
Table 2: CHN analysis of azo compounds.
|
Compd. |
Mol. formula |
Mol. weight |
Elemental analysis |
|||||
|
C% |
H% |
N% |
||||||
|
Cal. |
Found |
Cal. |
Found |
Cal. |
Found |
|||
|
A |
C14H11N3O5 |
301.25 |
55.82 |
55.53 |
3.68 |
3.90 |
13.95 |
13.57 |
|
B |
C14H11N3O5 |
301.25 |
55.82 |
55.90 |
3.68 |
3.63 |
13.95 |
14.20 |
|
C |
C15H12N2O5 |
300.27 |
60.00 |
60.17 |
4.03 |
4.14 |
9.33 |
9.62 |
|
D |
C14H11N3O6 |
317.25 |
53.00 |
52.81 |
3.49 |
3.57 |
13.24 |
13.35 |
|
E |
C14H11N3O6 |
317.25 |
53.00 |
52.75 |
3.49 |
3.41 |
13.24 |
12.51 |
|
F |
C15H12N2O6 |
316.27 |
56.96 |
57.13 |
3.82 |
4.02 |
8.86 |
9.10 |
|
G |
C14H11N3O5 |
301.25 |
55.82 |
55.61 |
3.68 |
3.45 |
13.95 |
13.68 |
|
H |
C14H11N3O5 |
301.25 |
55.82 |
56.03 |
3.68 |
3.51 |
13.95 |
13.73 |
|
I |
C15H12N2O5 |
300.27 |
60.00 |
59.83 |
4.03 |
3.85 |
9.33 |
9.49 |
FT-IR Spectra
The FT-IR spectra (KBr disc) of prepared compound show a strong absorption at (1654-1693 cm-1) for carbonyl carboxylic group.1,13 All compounds exhibit absorption bands at ranges (1604-1616 cm-1), (1523-1577cm-1) and (1438-1477 cm-1) for the stretching vibrations of -N=N- and C=C groups respectively because are superimposed in the same ranges.1,13 The spectra of the azo compounds show strong absorption bands at ranges (1273-1288 cm-1), (1195-1222 cm-1), (1249-1265 cm-1) and (1300-1354 cm-1) due to the stretching vibrations for (C-O, carboxylic), (C-O, phenolic), (C-N) and (NO2) respectively.1,13,14 The other FT-IR vibrations of the synthesized compounds are shown in Table (3).
1H-NMR Spectra
The proton-NMR analysis of prepared compounds performed by using deuterated dimethyl sulfoxide as a solvent. The 1H-NMR spectra of all prepared azo compounds showed a singlet signal within the ranges (2.264-2.296 ppm), (3.918-3.931 ppm) and (2.681-2.725 ppm) for the groups 3-CH3, 3-OCH3 and 4-CH3 respectively. Compounds A, B and C showed a singlet signals at (7.785-8.042 ppm) and (8.132-8.507 ppm) which attributed to protons H-4 and H-6 respectively. The 1H-NMR spectra of the synthesized compounds for compounds D, E and F showed singlet signals at ranges (7.675-7.732 ppm) and (8.050-8.444 ppm) for protons H-4 and H-6 respectively with 4J= 2.1. In addition to that compounds G, H and I appeared singlet signals at (7.013-7.072 ppm). Proton (H-6) for compounds G and H appeared at 8.171 ppm and 8.175 ppm respectively,15-17 the general structure of azo compounds was shown in Figure (3). Other aromatic protons of all azo synthesized compounds summarized in Table (4). The data of 1H-NMR spectra of synthesized compounds reported in Figures (4-12).
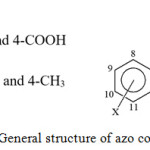 |
Figure 3: General structure of azo compounds. |
 |
Figure 4: 1H-NMR spectrum of azo compound A. |
 |
Figure 5: 1H-NMR spectrum of azo compound B. |
 |
Figure 6: 1H-NMR spectrum of azo compound C. |
 |
Figure 7: 1H-NMR spectrum of azo compound D. |
 |
Figure 8: 1H-NMR of azo compound E. |
 |
Figure 9: 1H-NMR spectrum of azo compound F. |
 |
Figure 10: 1H-NMR of azo compound G. |
 |
Figure 11: 1H-NMR of azo compound H. |
 |
Figure 12: 1H-NMR of azo compound I. |
Table 3: Data of FT-IR spectra (cm-1) of synthesized azo compounds.
|
Compd. |
C=O |
N=N C=C |
C=C |
C-O carboxylic str. |
C-O phenolic str |
C-N str. |
C-H Aromatic |
NO2 (Str.) sym. |
O-H Carboxylic |
O-H phenolic |
C-H aliphatic str. |
|
|
str, |
bend. |
|||||||||||
|
A |
1654 |
1527 1438 |
1608 |
1276 |
1222 |
1257 |
3039 3078 |
852 910 |
1346 |
2519 2600 |
3417 3587 |
2854 2989 |
|
B |
1658 |
1531 1446 |
1612 |
1284 |
1222 |
1257 |
3028 3082 |
848 910 |
1354 |
2525 2603 |
3417 3475 |
2870 2955 |
|
C |
1654 1685 |
1577 1446 |
1604 |
1288 |
1222 |
1265 |
3066 3160 |
867 906 |
1300
|
2596 2661 |
3414 3502 |
2866 2989 |
|
D |
1678 |
1580 1458 |
1609 |
1261 |
1230 |
1261 |
3082 |
887 914 |
1342 |
2645 |
3313 |
2997 |
|
E |
1667 |
1585 1469 |
1613 |
1285 |
1199 |
1267 |
3093 3194 |
879 898 |
1350
|
2603 2754 |
3421 3522 |
2870 2993 |
|
F |
1658 1680 |
1523 1454 |
1608 |
1276 |
1223 |
1277 |
3035 3100 |
860 910 |
1320 |
2620 2730 |
3522 3595 |
2854 2962 |
|
G |
1666 |
1527 1442 |
1608 |
1273 |
1219 |
1253 |
3074 3160 |
794 850 |
1342 |
3074 3159 |
3479 3552 |
2858 2927 |
|
H |
1685 |
1570 1477 |
1616 |
1276 |
1211 |
1249 |
3101 |
864 894 |
1346 |
2640 |
3414 3482 |
2840 2927 |
|
I |
1670 1693 |
1581 1485 |
1604 |
1284 |
1195 |
1250 |
3090 |
860 910 |
1342
|
2540 2700 |
3479 3552 |
2860 2910 |
Str. = stretching, bend. = bending, sym. = symmetrical.
Table 4: Data 1H-NMR for compounds A to I in DMSO-d6.
|
1H-NMR, δ (ppm), nJ H-H (Hz) |
|||
|
Comp. |
X’ |
C-H (aliphatic) |
(C-H) aromatic |
|
A |
3-CH3 |
2.264 (3H) |
7.785(H-4), 8.232(H-6), 7.952, (d, H-8, H-12, 3J8,9,3J12,10= 8.7), 8.371(d, H-9, H-11, 3J9,8= 3J11,12= 8.7) |
|
B |
3-CH3 |
2.296 (3H) |
8.042(H-4), 8.507(H-6), 7.876(t, H-9, 3J9,8= 3J9,10= 8.1), 8.266-8.373(m, H-8,H-10,H-12) |
|
C |
3-CH3 |
2.294 (3H) |
8.005(H-4), 8.132(H-6), 7.919(d, H-8, H-12, 3J8,9=J12,11= 8.4), 8.118(d, H-9, H-11, 3J9,8= 3J11,12= 8.4) |
|
D |
3-OCH3 |
3.918 (3H) |
7.675(d,H-4, 4J= 2.1), 8.063(d, H-6, 4J= 2.1), 8.045(d, H-8, H-12, 3J8,9= 3J12,11= 9), 8.409(d, H-9, H-11, 3J9,8= 3J11,12= 9) |
|
E |
3-OCH3 |
3.931 (3H) |
7.732(d,H-4, 4J= 2.1), 8.444(d,H-6, 4J= 2.1), 7.890 (t, 3J9,8= 3J9,10= 8.1), 8324-8.392(m, H-8, H-10), 8.650(d, H-12, 4J= 1.8) |
|
F |
3-OCH3 |
3.923 (3H) |
7.690(d, H-4, 4J= 2.1), 8.050(d, H-6, 4J=2.1), 7.952(d, H-8, H-12, 3J8,9= 3J12,11= 8.7), 8.130(d, H-9, H-11, 3J9,8= 3J11,12= 8.7) |
|
G |
4-CH3 |
2.725 (3H) |
7.072(H-3), 8.171(H-6), 8.066(d, H-8, H-12, 3J8,9= 3J12,11= 9), 8.412(d, H-9, H-11, 3J9,8= 3J11,12= 9) |
|
H |
4-CH3 |
2.725 (3H) |
7.066(H-3), 8.175(H-6), 7.880(t, H-9, 3J9,8=3J9,10= 8.1) 8.322-8.381(m, H-8, H-10), 8.532(H-12) |
|
I |
4-CH3 |
2.681 (3H) |
7.013(H-3), 7.913(d, H-8, H-12, 3J8,9, 3J12,11=7.5) 8.106 (3H, H-6, H-9, H-11) |
In vitro anti-inflammatory activity
In inflammation, there is extra cellular fluid release because cell damage, lysosome preventing the inflammation response if lysosomal membrane still stable. Lysosomal contain active enzyme like bactericidal enzyme and proteases, this cause additional tissue injury. So stability of lysosomal membrane very important for inflammatory prevention. The HRBCs membrane are used as analogous to lysosomal membrane, because they have similar components, a protection of HBRCs membrane of lysis were rated as anti-inflammatory activity.18
All the synthesized azo compounds (A-I) showed a significant anti-inflammatory activity by HRBC membrane stabilization method. The results of the activity are listed in Table (5).
Table 5: Protection of HRBC membrane of compounds at dose 75 mg.
|
The compound |
Protection % |
|
A |
20.16 |
|
B |
69.53* |
|
C |
31.86 |
|
D |
28.30 |
|
E |
71.87* |
|
F |
25.15 |
|
G |
23.95 |
|
H |
79.76* |
|
I |
30.43 |
|
Sodium diclofenac |
74.48* |
* = p< 0.01
From the results, compounds B, E and H show high activity compared with other compounds. The active compounds B, E, and H contain a nitro group at meta position. Replacing nitro group with carboxyl group will be minimize the activity. Therefore, the activity is the best when a nitro group located at meta position. In salicylic acid moiety, the activity of protection was found increased when methyl group located at para– position compared with the meta-methyl and meta-methoxy groups. Compound E contain meta nitro group and meta methoxy group, it is the compound with meta-methoxy group has high activity than other methoxy derivatives. The results showed compounds A, D, F and I have less activity. The action of the azo compounds could be related to the binding of compounds with erythrocyte membrane, especially phospholipids. Since the compounds has polar and nonpolar groups can bonded with same groups of phospholipids. This prevents membrane damage by physical interaction of osmotic pressure differences, which is causative to hemolysis of red blood cells.19
Conclusion
In this study, three series of azo were prepared using three different of salicylic acid derivatives. The prepared compounds identified using the element analysis (CHN) as well as infrared and 1H-NMR spectroscopy. The results supported the structures of prepared compounds. All compounds showed significant in vitro anti-inflammatory activity. Compounds B, E and H showed high anti-inflammatory activity compared with other prepared azo compounds.
Conflict of Interest
There is no conflict of interest.
References
- Potey, L. C.; Urade, P.; Aate J.; Kosalge, S., Int. J. ChemTech. Res. 2017, 10, 552-556.
- Otutu, J. O.; Okoro, D.; Ossai, E. K.; J. App. Sci. 2008, 8, 334-339.
- Patil,C. J.; Nehete, C. A.; Int. J. Pharm. Sci. Rev. Res. 2015, 33, 248-256.
- Swati, Ginni, Karnawat, R.; Sharma, I. K.; Verma, P. S.; Int. J. of Applied Biology and Pharmaceutical Technology, 2011, 2, 332-338.
- Khalid, A.; Arshad, M.; Crowley, D. E.; Appl. Microbiol Biotechnol 2008,78, 361-369. DOI: 10.1007/s00253007-1302-4.
- Farghaly, T. A.; Abdallah, Z. A.; ARKIVOC 2008, (xvii), 295-305. http://dx.doi.org/10.3998/ark.5550190.0009.h28.
- Dardeer, H. M.; Elboray, E. E.; Mohamed, G. S.; Polycyclic Aromatic Compounds 2018, 1-11. https://doi.org/10.1080/10406638.2018.1466812
- Fadey, O. O.; Obafemi, C. A.; Adewunmi, C. O.; lwalewa, E. O.; African Journal of Biotechnology 2004, 3, 426-431.
- Neha, Patni, M.; Der Chemic Scienca 2016, 7, 93.
- Ahmadi R. A.; Amani, S.; Molecules 2012, 17, 64346-448. Doi: 10.3390/molecules17066434
- Koshti, S. M.; Sonar, J. P.; Sonawane, A. E.; Pawar, Y. A.; Nagle, P. S.; Mahulikar, P. P.; More, D. H.; Indian J. Chem. 2008, 47B, 329-331.
- Solomon, S.; Senthamilselvi, M. M.; Muruganantham, N.; J. of Bioscience and Applied Research 2016, 2, 621-625.
- Sahoo, J.; Paidesetty S. K.; Egypt. J. of basic and applied sciences 2015, 2, 268-280. http://dx.doi.org/10.1016/j.ejbas.2015.07.006.
- Rathod, K. M.; Thakre, N. S.; Chem Sci Trans. 2013, 2, 25-28.
- Zhao, Z. B.; Zheng, H. X.; Wei, Y. G.; Liu, J.; Chinese Chemical Letter 2007,18, 639-642.
- da Silva, M.; Menezes, C. M. S.; Ferreira, E. L.; Leite, C. Q. F.; Sato, N.; Pimenta, C. P.; Botelho, K. C. A.; Chem. Biol. Drug Des. 2008, 71, 167-172.
- A Jilani, J.; shomaf, M.; alzoubi, K. H., Drug Design, Development and Therapy, 2013, 7, 691-698.
- De, P.; Sarkar, S.; Mukhophadhyay, M. J.; J. of Pharmacognosy and Phytochemistry 2017, 6, 103-105.
- Oyedapo, O. O.; Akinpelu, B. A.; Akinwunmi, K. F.; Adeyinka, M. O.; and Sipeolu, F. O.; Inter. J. Plant Physiology and Biochemistry 2010, 2, 46-51.

This work is licensed under a Creative Commons Attribution 4.0 International License.









