Synthesis of Chitosan from the Scales of Starry Trigger Fish (Abalistes Stelaris)
1Department of Rubber and Plastic Processing Technology, Politeknik ATK Yogyakarta, Indonesia.
2Department of Leather Processing Technology Politeknik ATK Yogyakarta, Indonesia.
Corresponding Author E-mail: swatika.rustiawan@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350147
Article Received on : 01-11-2018
Article Accepted on : 28-11-2018
Article Published : 02 Jan 2019
Synthesis of chitosan from the scales of starry trigger fish has been carried out in three stages: deproteination using 10% NaOH solution, demineralization using 0.63 M HCl solution, and deacetylation using NaOH solution with three different concentration of NaOH, those were 60%, 50%, and 40% NaOH. Characterization of synthesized chitosan showed that the best chitosan was obtained by deacetylation process with 60% NaOH. The degree of deacetylation obtained 74% has met the DD standard of at least 70% chitosan. Ash level 88.05%; 6% water content; 2.29% protein content.
KEYWORDS:Chitosan; Deacetylation Degree; Starry Trigger Fish
Download this article as:| Copy the following to cite this article: Hermiyati I, Iswahyuni I, Juhana S. Synthesis of Chitosan from the Scales of Starry Trigger Fish (Abalistes Stelaris). Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Hermiyati I, Iswahyuni I, Juhana S. Synthesis of Chitosan from the Scales of Starry Trigger Fish (Abalistes Stelaris). Orient J Chem 2019;35(1). Available from: https://bit.ly/2BB1M4v |
Introduction
The use of biopolymers has developed in all aspect, one of biopolymer is chitosan which is use as biopreservative, emulsificator, stabilizator.1 Chitosan can be derived from Chitin which is synthezed from solid waste of crustaceans such as shells, heads, feet and scales, and from scales of some fishes.2 By using the waste product as cheap and readily found in natural resources hopefully would solve the problems of solid waste in the seafood processing industry. One of fish that can be use as natural natural source is the scales of starry trigger fish (Abalistes stelaris).
The increasing consumption of starry trigger fish is followed by the increase solid wastes such as skin, bone and scales of the fish. The physical properties of starry triggerfish are very hard outer skin and the scales cannot be cleaned with scales cleaning tool. Due to those uninteresting physical properties, the consumers only consume the flesh; while the skins as well as the scales are discarded around the beach thus became solid wastes. The amount of starry trigger fish skin that were discarded in around Tasik Agung beach, Rembang district, Central Java, Indonesia reached about 25 to 50 kg per day or 50-1500 kg per month. This waste product of fish skin has been utilized to produce valuable leather products by tanning using vegetable tanning process.3 Now, the skin of this fish has been tried to synthesize chitosan and characterize by FTIR to determine the formation of NH2 groups in chitosan and to calculate the deacetylation degree of chitosan. The use of the scales of starry trigger fish to produce chitosan will increase valuable products and helps to overcome solid wastes around the beach.
Chitosan can be syntheses from chitin which is extracted from some scales fish.2 Chitin was first discovered in 1811 by Henry Braconot by extracting certain types of fungi using water, alcohol and dilutes alkalis that produce insoluble deposits called fungi. The extraction results as a pure form of cellulose although the presence of nitrogen is unknown. Then in 1823 Odie found the same component in the insect ectodermal (cuticle) and named it chitin from the Greek word “chiton” which means cover or cover.
Chitin is composed of units of acetyl glucosamine which bind to each other, with (1-4) beta bonds. Chemically chitin is a polysaccharide group polymer composed of units of β- (1-4) 2-acetamide-2-deoxy-D-glucose, which can be digested by mammals. Chitin is a formless solid (amorphous), insoluble in water, dilutes organic acids, dilute and concentrated alkalis, alcohol, has a physical form in the form of crystals which are white to light yellow, tasteless, and odorless with the molecular formula [C6H13O5N] ¬n. The molecular weight of chitin is very large depending on the length of the chain. From the molecular formula above, the molecular weight is [203,19] n. Chitin has no melting point, and has a nitrogen content of less than 7%. Chitin is obtained from scales waste through deproteinization and demineralization processes. Deprotenation is the process of removing protein, which is extracted with NaOH solution. In principle the deproteinization process is to separate or release bonds between protein and chitin. This process is generally carried out with treatment using NaOH solution at relatively high temperatures for a relatively long time. With this treatment protein will be released and form soluble Na-proteinate. Demineralization is a stage in the removal of minerals which serves to separate chitin from inorganic salts by adding dilute HCl at room temperature, so that the existing minerals will react with HCl.4 The reactions that occur are as follow:
CaCO3 (s) + 2 HCl (s)CaCl2 (l) → + H2O (g) + CO2 (g)
Ca3(PO4)2 (s) + 6 HCl(l) → 3CaCl2 (l) + 2H3PO4
Treatment with dilute hydrochloric acid at room temperature is more effective and produces chitin with a lower residual mineral content, although it can cause breaking of the chain or the release of its acetyl groups. Chitosan is chitin which is removed by acetyl group, so this material is a polymer of glucosamine which is also called β-1,4-2, amino-2-dioxy-D-glucose. (Muzarelli, 1985 in Suhardi, 1992).5 Chitosan is white, non-toxic, odorless, easily biodegradable, and slightly soluble in HCl, HNO3 and H3PO4. The free amino groups they contain make this polymer polycationic so it is not soluble in water and dissolves in strong bases. Chitosan is also insoluble in organic solvents such as alcohol, acetone, dimethylformamide, dimethylsulfoxide, but is easily soluble in organic acids such as citric acid and high concentrated formic acid (0.2% -100%) in water.6 Besides that chitosan easily interacts with organic substances such as proteins, so it is relatively more widely used in various applied industries and the health industry.
Chitosan has the same chemical structure as chitin, consisting of long molecular chains and large molecular weight. The difference between chitin and chitosan is that in each ring of chitin molecules there is an acetyl group (CH3-CO-) on the second carbon atom, whereas in chitosan there is an amine group (NH2–). Chitosan contains more than 7% nitrogen (Fig 1).7
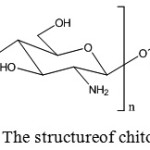 |
Figure 1: The structureof chitosan. |
Chitosan can be produced from chitin by deacetylation process by reacting using high concentration alkali with relatively long time and high temperature, by removing acetyl group (-COCH3) so that the molecule can dissolve in acid solution. This process is called deacetylation, which releases acetyl groups so that chitosan has the characteristics of a cation. In general, the deacetylation degree of chitosan is around 60% and around 90-100% for chitosan which has full deacetylation, this price depends on the chitin raw material used and the process carried out.4
The degree of deacetylation was influenced by alkaline solution concentration, temperature, reaction time, chitin pre-treatment, particle size and chitin concentration.8
One of the quality parameters of chitosan is the degree of deacetylation. The degree of deacetylation is a parameter of chitosan quality which shows the percentage of acetyl groups that can be removed from chitin yield. The higher the deacetylation degree of chitosan, the lower the acetyl chitosan group so that the interaction between ions and hydrogen bonds becomes stronger. The more amine groups in chitosan also cause chitosan to become more reactive. The quality parameters of chitosan according to9 are shown in Table 1.
Table 1: The quality parameters of chitosan.
| Quality Parameters | Standard value of chitosan |
| Particle size | Pellets |
| Ash content (%b/b) | <1.0 |
| Moisture content (%b/b) | <10.0 |
| Solubility in an acidic atmosphere | > 95.0 for pharmacy types |
| Color | Only at pH <6 |
| Deacetylation degree (%) | > 70.0 for technical types |
Materials and Methods
Research Materials
The scales of starry trigger fish as waste product were collected from the fishermen in Tasik Agung beach, Rembang district, Central Java Province, Indonesia. The reagent and chemicals used in this study were such as Sodium Hydroxide (NaOH), Cloride Acid (HCl), and pH indicator paper. The chemicals were`purchased from Merck.
Pretreatmen of Raw Material
The scales of starry trigger fish were cleaned by washing in running water, then they were dried under the sun. The dried fish scales were mashed to a size of 100 mesh.
Isolation of Chitin
Prior to chitin isolation the scales of starry trigger fish were cleaned by washing in running water, and thenthey weredried under the sun. The dried fish scales were mashed to a size of 100 mesh. Chitin isolation was carried out through deproteinization stage by refluxing 50 grams of the scales powder with 500 ml of 4% (w/v)NaOH solution at 65oC for 2 hours, then it was refluxed with distilled water until the pH reached neutral and the resulting filtered was dried in the oven. After the deproteination the process was continued with the demineralization process, the residue plus 0.5M HCl with a ratio of 1:15 was stirred at room temperature for 3 hours. The aim of demineralization was to remove minerals (mainly CaCO3).10 Then the mixture was filtered and washed with distilled water until the pH was neutral. The washing residue was heated in the oven, and it was called chitin.
Synthesis of Chitosan
Synthesis of chitosan was carried out through a deacetylation process. Fifty grams of chitin were refluxed with three different concentration of NaOH with a ratio of 1:10 at 70oC for 1 hour. Concentration of NaOH for deacetylation is chitosan 1 with 60% NaOH, chitosan 2 with 50% NaOH, chitosan 3 with 40% NaOH. The reflux product was cooled and filtered to a neutral washing pH. The residue was then heated using an oven and the chitosan was obtained. The chitosan obtained will be in a creamy white form.11
Characterization of Chitin and Chitosan
The characterization of chitin and chitosan was carried out by using the described method12 including moisture content, ash content, protein content and deacetylation degree.
Moisture Content
One gram of chitosan was put in a porcelain cup then it was heated in the oven at 105oC for 3 hours, then it was cooled in a desiccator and weighed. The water content of chitosan was calculated by calculating the moisture content (%) was calculated by using this formula: (weight of chitin or initial chitosan (gram) -weight of chitin or final chitosan (gram))/ weight of chitin or initial chitosan (gram) x 100%.
Ash Content
One gram of dried chitin or chitosan was put into a cup, then burned on an electric stove until no longer smoky and then put in the furnace for 5 hours at 650oC. The cup was cooled in a desiccator then weighed. Ash content was calculated as follows: (Weight of ash/weight of sample) x 100%.
Protein Content
Protein content analysis consists of three stages: destruction, distillation and titration. A total of 0.25 grams of chitin or chitosan were added to 100 mL of Kjeldahl flask and added with one kjeltab and 3 mL of concentrated H2SO4. The mixture was destructed at 410oC for 1 hour until the solution was clear and cooled down. The mixture in kjeldahl flask was added with 50 mL of distilled water and 20 mL of 40% NaOH and distilled at 100oC. The distilled water in an Erlenmeyer flask was added with a mixture of 2 mL of 2% boric acid (H3BO3) and 2 drops of bromocresol green-methyl red indicator. The distillation process was stopped when the volume of distillate reached 40 mL and the color was bluish green. The distillate was then titrated with 0.1N HCl until a pink color was formed. Protein levels were determined by calculating nitrogen content (%) using this formula: ((mL HCl-mL blank) x N HCl x 14,007/ mg sample) x 100%.
The Degree of Deacetylation (DD)
FTIR spectrum chitosan synthesized from starry trigger fish scales were recorded using FTIR spectrophotometer (FT-IR 8400S, Shimadzu, Japan). The degree of deacetylation (DD) was determined by infrared spectrophotometry methods. The deacetylation degree of chitin and chitosan can be calculated based on the comparison of the absorbance of amide groups (about 1655 cm-1) with hydroxyl groups (about 3450 cm-1). The absorbance ratio was calculated after firstly determined the baseline in each spectra so as to produce two different baselines in the absorption area of 1655 cm-1 namely baseline A and B.13
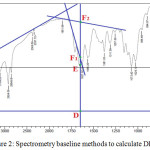 |
Figure 2: Spectrometry baseline methods to calculate DD. |
Figure chitosan 2 shows the chitosan FTIR spectra from the deacetylation process with 50% NaOH. The spectra results show there was an -OH group of chitosan groups which were shown in wave numbers 1412.87 cm-1 with low intensity, -CH groups at wave numbers 1469 cm-1, –CO groups at wave numbers 1025.75 cm-1, CH-alkene groups at wave number 872.70. The calculation of deacetylation degree for this chitosan wass 47.58%.
The formula for determining baseline A based on the Domzy and Robert formula14 is as follows:
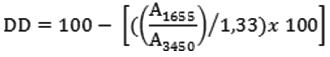
The area of absorbance of amine groups and hydroxyl groups can be presented further by the mathematical equations proposed by Sabnis and Block as bellow:
log(DF1/DE) = A1655
log(DF2/DE) = A1655
log(AC/AB) = A3450
DF1 (used for baseline A) or DF2 (used for baseline B), DE, AC and B show the absorbance band of the functional group as the wavelength region.
Results and Discussion
The results of chitosan rendement testing stated the efficiency of chitosan isolation process from the scales of starry trigger fish. The randement calculation was obtained from the weight of chitosan compared to the weight of the powder of starry trigger fish scales as shown in Table 2.
Table 2: Characteristic of chitosan synthesized from the scales of starry trigger fish based on yield by using three different concentrations of NaOH.
| No | Process | Concentration of NaOH (%) | Initial Weight (gram) | Final Weight (gram) | Yield (%) |
| 1 | chitosan 1 | 60 | 20 | 3.00 | 15.0 |
| 2 | chitosan 2 | 50 | 30 | 16.05 | 53.5 |
| 3 | chitosan 3 | 40 | 54 | 16.24 | 30.0 |
Further characteristic of synthesized chitosan is listed in Table 3.
Table 3: Characterization of water, ash and protein content of chitosan synthesized from starry trigger fish.
| No | Process | Water Content (%) | Ash Content (%) | Protein Content (%) |
| 1 | chitosan 1 | 6.02 | 88.50 | 2.29 |
| 2 | chitosan 2 | 3.43 | 87.67 | 1.07 |
| 3 | chitosan 3 | 5.05 | 91.80 | 1.08 |
The Table 3 shows that the water content and protein content of all chitosan were below 10%, however, ash content for the three chitosan were still very high indicated the high content of mineral in the scales of starry trigger fish. The hardness of the scales of starry trigger fish indicated the high content of mineral component of materials. The higher mineral content the harder mineral component of the materialTalumepa, et al (2016).15 Asbiopersepatif, chitosan must have water content below 10%. So this synthesis chitosan is good water content because all chitosan have water content below 10%. With low water content so microorganisme can not life in chitosan.
Characterization of synthesized chitosan was carried out by FTIR to determine the formation of NH2 groups in chitosan and to calculate the deacetylation degree of chitosan. The results of spectrometry from FTIR are shown in Figures 3.
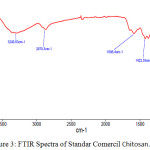 |
Figure 3: FTIR Spectra of Standar Comercil Chitosan. |
Figure 3 shows the chitosan FTIR spectra from standar chitosan comercil. Comercil chitosan is a chitosan that is sold in general. The bonds in the FT-IR spectra of standar chitosan is -OH stretching 3858 cm-1, -NH stretching 3609 cm-1, -CH stretching 2862 cm-1, amide 1 band 1643 cm-1, amide 2 band 1552 cm-1, CH2 bending 1421 cm-1, CO-stretching 1022 cm-1, CH3 wagging alone chain 752 cm-1.16
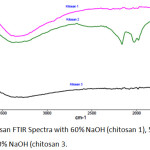 |
Figure 4: Chitosan FTIR Spectra with 60% NaOH (chitosan 1), 50% NaOH (chitosan 2), 40% NaOH (chitosan 3. |
Figure 4 shows the chitosan FTIR spectra from the deacetylation process with 60% NaOH (chitosan 1), 50% NaOH (chitosan 2), and 40% NaOH (chitosan 3). Characteritation of comercil chitosan, chitosan 1, 2, 3 shows Table4.
Table 4: Characteritation of chitosan 1, 2, 3.
| Vibration | Wave Number (cm-1) | |||
| Comercil Chitosan (Fig.3) | Chitosan 1 | Chitosan 2 | Chitosan 2 | |
| -O-H | 1422,38 | 1419 | 1412.87 | 1415,7 |
| -N-H streching | 3290,53 | 3353,45 | 3380,35 | 3326,7 |
| -C-O | 1024,67 | 1023 | 1025,75 | 1020,28 |
| -N-H bending | 1586,4 | 1651 | 1635,8 | 1646,1 |
| -C-H group | – | – | 1469 | 1460,8 |
| -C-H alkene group | – | – | 872,70 | 871,05 |
| -C-H strecting | 2870,3 | – | 2163,01 | – |
Table 4 shows chitosan 1,2,and 3 have FTIR spectra like comercil chitosan. From the chitosan spectra FTIR, the degree of deacetylation can be calculated based on the ratio of the absorbance of the amida group and the hydroxy group. The calculation of deacetylation degree for this chitosan 1 was 74,25%. The calculation of deacetylation degree for this chitosan 2 wass 47, 58%. The calculation of deacetylation degree for this chitosan 3 wass 40, 19%. Deacetylation Degrees calculated based on Domzy and Robert formula14 in Saleh, et al (2015) are listed in Table 5.
Table 5: Deacetylation Degree (DD) of chitosan synthesized from the scales of starry trigger fish.
| No | NaOH Concentration in the Deacetylation Process | Deacetylation Degree(%) |
| 1 | 60% (Chitosan 1) | 74.00 |
| 2 | 50% (Chitosan 2) | 47.58 |
| 3 | 40% (Chitosan 3) | 40.19 |
The deacetylation degree (DD) value of chitosan 1 was 74%, met the standard minimum quality parameter of chitosan DD which was 70% (Bastaman, 1989 in Jumadi, 2006)9. The calculation results of DD according Laboratory Protan that DD of chitosan ranges from 70-100%.17 DD of chitosan 2 and 3 not according DD of standar chitosan. This was because the NaOH with concentration 50% and 40% in the deacetylation degree was not able to remove the acetyl group in chitin.
Conclusion
The scales of starry trigger fish as west product in fishery industry is a good material for chitosan synthesis which used NaOH for deacetylation process. Ash content of chitosan synthesized by this process is still high due to the very hard character of scales of starry trigger fish. The 60% of NaOH is the best concentration to obtain chitosan with 74% DD which met the standard DD of material.
Competing Interests
We declare that we have no competing interests.
Acknowledgements
The authors acknowledge the kindness and co-operation of the informal and the local administration officer in Tasik Agung beach, Rembang district, Central Java Province, Indonesia. This research was supported by the Politeknik ATK Yogyakarta, Indonesia.
References
- Knorr, D. Use of Chitinous Polymers in Food—A Challenge for Food Research and Development. Food Technology., 1984, 38, 85-97.
- Azis, N.; Gufran, M.F.B.; Pitoyo,W.U.; Suhandi., Hasanuddin Student Journal Vol., 2017, 1(1), 56-61.
- Hermiyati, I.; Sya’bani, M.W.; Silvianti, F., The 7th International Seminar on Tropical Animal Production., 2017, 473-484.
- Suhardi. Book of Monograf Chitin dan Chitosan. Pusat Antar Universitas , Food and Nutrition, Universitas Gadjah Mada. 1992, Yogyakarta.
- Muzarelli, R.A.A. Chitin In GO Aspinall, Polysaccharides., 1985. (Vol 3). Academic Press. New York.
- Sugiyo.The Study of the Use of Green Crab (Scylla serrata) as an Adsorbent for Cobalt (III) and Nickel (II) Metal Ions in Medium Water. Tesis. 2001, Jurusan Kimia FMIPA UNY, Yogyakarta.
- Muzzarelli, RAA. Chitin. 1977. Chitin. Pergamon Press. Oxford, UK.
- Ahlafi, H; Moussout, H; Boukhlifi, F; Echetna, M; Bennani, M.N; and Slimane, S.M, Mediterranean Journal of Chemistry., 2013. 2(3), 503-513.
CrossRef - Bastaman, S. Studies on degradation and extraction of chitin and chitosan from prawn shells (Nephrops norregicus). 1989. Thesis. The department of Mechanical Manufacturing, Aeronautical and Chemical Engineering. The Queen’s University of Belfast. England.
- Trung, T.S; Thein-Han, W.W; Qui, N.t; Ng, C.H; Stevens, W.F., BioresourTechnol., 2006, 97(4), 659-663.
CrossRef - Muzzarelli, R.A.A; Rochetti,R. Journal CarbohydrPolym., 1985,5, 46177.
- (AOAC) Association of Official Analytical Chemist., Official Methods of Analysis (18Edn)., The Association of Official Analytical Chemist. Inc. 1999, Mayland. USA.
- Khan, S.H. ; Bhatti, B.M., Pakistan Vet. J., 2002, 22 (1), 27-30.
- Saleh, A; Mukhtar, S.A; Fawwaz, M; Pratama, M., Journal of Chemical and Pharmaceutical Research, 2015, 7(11), 265-269.
- Talumepa, A.C.N.; Suptijah, P.; Wullur, S.; Rumengan, I.F.M., Jurnal LPPM Bidang Sains dan Teknologi., 2016, Volume 3 Nomor 1.
- Puvvada, Y.S; Vankayalapati, S; Sukhavasi, S.,Int Curr Pharm J., 2012, 1(9):258-263.
CrossRef - Ifa, L; Artiningsih, A; Julniar; Suhaldin., Journal of Chemical Process Enginering., 2018, 03(01):47-50.

This work is licensed under a Creative Commons Attribution 4.0 International License.










