Synthesis and Antimicrobial Activity of Novel Pyrazole Derivatives
Department of Chemistry, Faculty of Science, Albaha University, Saudi Arabia.
Corresponding Author E-mail: hassan11220@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350149
Article Received on : 25-10-2018
Article Accepted on : 20-01-2019
Article Published : 19 Feb 2019
This work aimed to synthesis some new different heterocyclic compounds based on phenylpyrazole-4-carbaldehyde derivative (1) reacted with cyanoacetohydrazide to afford N'‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐2‐cyanoacetohydrazide (2).compound (2) cyclized by acetyl acetone, and aromatic aldehyde, respectively, to give pyrazolo-pyridone (4) and substituted pylazolochromene (6 and 8), respectively. Pyrazolo triazole (10) was obtained by coupling of 1-azido-4-fluorobenzene with (2), which was diazotized by 3‐[(E)‐2‐chlorodiazen‐1‐yl]‐4,6‐dimethyl‐1H‐pyrazolo[3,4‐b]pyridin to give pyrazolo pyridine derivative (11). N,N-Dimethylformamide dimethyl acetal reacted with (2) to afford (2E)‐N'‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐2‐cyano‐3‐(dimethylamino)prop‐2‐enehydrazide (13). This compound hyadrazonlysis to yield substituted pyrazole-4-carbohydrazide (15). Also, compound (2) reacted with phNCS in presence of KOH and furnish compound (22), which preferred over compound (20) All synthesized compounds were established using IR, 1H-NMR. These compounds (2,3,6,8,12,13,15 and 22) were screened against several microorganisms to investigate antimicrobial potency.
KEYWORDS:Chromene; Pyrazole; Pyridine And Antimicrobial Activity; Thiazole
Download this article as:| Copy the following to cite this article: Al-Ghamdi H. M. Synthesis and Antimicrobial Activity of Novel Pyrazole Derivatives. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Al-Ghamdi H. M. Synthesis and Antimicrobial Activity of Novel Pyrazole Derivatives. Orient J Chem 2019;35(1). Available from: https://bit.ly/2IoRHxj |
Introduction
Pyrazolos have attracted considerable attention for many researcher, due to their broad effects in many different fields,1-2 as agrochemicals,3-4 antimicrobial5 antifungal,6 anticancer,7 antidiabetic,8 anti-inflammatory,9 anticancer,10 antiviral,11 anti-HIV activity.12,13 Moreover, pyrazolo-chromene derivatives were exhibited powerful antimicrobial and antifungal activities14-15 Also, pyrazole compounds based on pyridone ring moiety were evaluated against their antibacterial and antifungal activities pyrazolpyridone derivatives exhibit positive acceptance as antimicrobial character.16-17 As well as, when introducing pyrazole and thiazole fragments to parent pyrazole were enhancement antimicrobial power.18-21 Thus, this work aimed to prepare the novel heterocyclic compounds based on pyrazole moiety and investigate their biological evaluation as antimicrobial and antifungal activities.
Materials and Methods
All compounds have obtained from Sigma-Aldrich and used without future purifications. Recorded Melting points for synthesized compounds are inaccurate. recorded IR spectrum (KBr) were measured on FTIR-5300 spectrometer (n max, cm-1). Brucker Ultra-Shield 850 MHz was used for generated 1H-NMR spectra in (CDCl3 & DMSO-d6) and reported in (ppm).
N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]-2-cyanoaceto hydrazide (2)
A (0.02 mol) of aldehyde (1), was refluxed with 2-cyanoacetohydrazide (0.02 mol), in presence of (60 ml) absolute ethanol for 5 h at 120°C. The reaction solution was left in air for 24 hrs., and the solid product collected, dry out. m.p.: 188-190°C, yield: 5.3 g (88%), pale yellow (acetic acid). IR: 1682 (CO), 2263 (CN), 3196 (NH). 1H-NMR: δ = 2.55 (s, 3H, CH3), 3.85 (s, 2H, CH2), 7.26 – 7.84 (m, 5H, Ar-H), 7.84 (s, 1H, CH), 9.60 (s, 1H, NH). M.F. C14H12ClN5O. Anal./Calcd: C( 55.73); H (4.01); Cl(11.75); N(23.21); O( 5.30). Found: C(55.70); H(3.38); Cl(11.79); N(23.26); O(5.28).
Preparation of 1‐[(Z)‐[(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]amino‐4,6‐dimethyl‐2‐oxopyridine‐3‐carbonitrile (4)
This compound was prepared by a modification to that previously reported method.22 A (0.002 mol) of pyrazole derivative (2), (0.002 mol) of acetyl acetone in (25 ml) of absolute ethanol and catalytic amount of piperidine was added up. The response solution was wormed at 180°C for 3 h. The desired solid product appeared by refrigeration, then filtered off, washed with methanol, and dried. m.p.: 235-237°C, yield: 0.6 g (82%), yellow (benzene). IR: 1652 (C=O), 2216 (CN) .1H-NMR: δ = 2.43 (s, 6H, 2 CH3), 2.58 (s, 3H, CH3), 6.09 (s, 1H, CH pyridine), 7.26 – 7.55 (m, 5H, Ar-H) and 8.94 (s, 1H, CH). M.F. C19H16ClN5O. Anal./Calcd: C(62.38); H(4.41); Cl (9.69); N(19.15); O(4.37). Found: C(62.32); H(4.37); Cl(9.71); N(19.18); O(4.35).
Reaction of Pyrazole-2-Cyanoacetohydrazide with Aromatic Aldehyde Derivatives
A (0.002 mol) of compound (2) was reacted with aromatic aldehyde derivative (0.002 mol), in absolute ethanol (25 ml) and 0.3 ml of piperidine was added. The response reaction solution was heated for 2-3 h at 120°C. The reaction mixture cooled, and the desired compound was collected, then dried.
N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐2‐iminochromene‐3‐carbohydrazide (6a)
m.p.: 236-238°C, yield: 0.68 g (85%), yellow (ethanol/benzene). IR: 1688 (CO), 3053, 3296 (NH). 1H-NMR: δ = 2.61 (s, 3H, CH3), 7.12 – 7.78 (m, 10H, Ar-H, chromene-H) 7.79 (s, 1H, CH), 10.01 (s, 1H, NH), 13.49 (s, 1H, NH chromene). M.F. C21H16ClN5O2. Anal./Calcd: C(62.16); H(3.97); Cl(8.73); N(17.26); O(7.88). Found: C(62.13); H(3.96); Cl(8.77); N(17.25); O(7.89).
N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐2‐imino‐6‐methoxychromene‐3‐carbohydrazide (6b)
m.p.: 270-272°C, yield: 0.7 g (81%), orange (acetic acid). IR : 1695 (CO), 3055 , 3239 (NH). 1H-NMR : δ = 2.71 (s, 3H, CH3), 3.98 (s, 3H, CH3 Methoxy), 7.15 – 7.59 (m, 9H, Ar-H, chromene-H), 8.30 (s, 1H, CH), 9.04 (s, 1H, NH), 11.83 (s, 1H, NH chromene-H). M.F. C22H18ClN5O3. Anal./Calcd C(60.62); H(4.16); Cl(8.13); N(16.08); O(11.01). Found: C(60.55); H(4.12); Cl(8.14); N(16.10); O(11.04).
6‐bromo‐N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐2‐iminochromene‐3‐carbohydrazide (6c).
m.p.: 248-250°C, yield: 0.82 g (86%), yellow (ethanol). IR: 1681 (CO), 3213 (NH). M.F. C21H15BrClN5O2. Anal./Calcd: C(52.03); H(3.12); Br(16.48); Cl(7.31); N(14.45); O(6.61). Found: C(51.98); H(3.11); Br(16.42); Cl(7.32); N(14.43); O, (6.60).
Preparation N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐3‐iminobenzo[f]chromene‐2‐carbohydrazide (8).
A (0.002 mol) of compound (2) was cyclized by reaction of (0.002 mol) 2-hydroxynapthaldehyde in (20 ml) ethanol media with few drops of piperidine. The desired solution was warmed at 15°C for 3 h, the desired compounds was collected by filtration ,then purified with cold methanol, and dried. m.p.: 242-244°C, yield: 0.73 g (80%), pale yellow (ethanol/benzene) IR: 1689 (CO), 3049, 3242 (NH). M.F. C25H18ClN5O2. Anal./Calcd: C(65.86); H(3.98); Cl(7.78); N(15.36); O(7.02). Found: C(65.78); H(3.94); Cl(7.76); N(15.38); O(7.01).
Synthesis of 5‐amino‐N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐1‐(4‐fluorophenyl)‐1,2,3‐triazole‐4‐carbohydrazide(10)
1-azido-4-fluorobenzene (0.001 mol) was dissolved in absolute ethanol (20 ml). And (0.001 mol) of compound (2), in presence of 1.2 g of sodium ethoxide was added. Then reaction solution refluxed at 175°C for about 3hrs., the desired compounds was collected by filtration ,then purified with cold ethanol,. m.p.: 280-282°C, yield: 0.33 g (75%), white (1,4-dioxane). IR: 1640 (CO), 3275 (NH), 3422, 4552 (NH2). 1H-NMR : δ = 2.51 (s, 3H, CH3), 6.59 (s, 2H, NH2), 6.46 – 7.71 (m, 9H, Ar-H), 8.57 (s, 1H, CH), 11.91 (s, 1H, NH). M.F. C20H16ClFN8O. Anal./Calcd: C(54.74); H(3.68); Cl(8.08); F(4.33); N(25.50); O(3.65). Found: C(54.69); H(3.64); Cl(8.10); F(4.34); N(25.57); O(3.62).
Synthesis of (Z)‐1‐{N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4-yl)methylidene]hydrazinecarbonyl}‐N‐{4,6‐dimethyl‐1H‐pyrazolo[3,4‐b]pyridin‐3‐yl}methanecarbohydrazonoyl cyanide (11)
(0.001 mol) of pyrazole derivative (2) and 3‐[(E)‐2‐chlorodiazen‐1‐yl]‐4,6‐dimethyl‐1H‐pyrazolo[3,4‐b]pyridine (0.001 mol), in pyridine (15 ml) was stirred at 0˚C for 3 h, then put into cold water. The solid product was filtered and dried. m.p.: 244-246˚C, yield: 0.4 g (85%), green (ethanol/benzene). IR: 1652 (CO), 2217 (CN), 3212, 3262 .3403 (NH). 1H-NMR: δ = 2.70 (s, 3H, CH3), 2.92 (s, 3H, CH3), 3.18 (s, 3H, CH3), 7.15 – 7.59 (m, 6H, Ar-H), 8.28 (s, 1H, CH), 8.28 (s, 1H, NH), 9.35 and 11.03 (s, 2H, NH). M.F. C22H19ClN10O. Anal./Calcd: C(55.66); H(4.03); Cl(7.46); N(29.48); O(3.37). Found: C(55.57); H(3.98); Cl(7.47); N(29.51); O(3.36).
General procedure for Reaction of pyrazole-2-cyanoacetohydrazide with benzenediazonium chloride derivatives.
(0.001 mol) of Pyrazole derivative (2) and 1-chloro-2-phenyldiazene derivative (0.001 mol) was liquified in (15 ml) of pyridine. The desired solution was left for 2hrs. under stirring condition at 0°C.then put into cold water. The solid product was filtered and collected.
(E)‐1‐{N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)met-hylidene]hydrazinecarbonyl}‐N‐phenylmethanecarbo-hydrazonoyl cyanide (12a)
m.p.: 196-197°C, yield: 0.35 g (87%), red (ethanol). IR : 1667 (CO), 2220 (CN), 3042 , 3256 (NH). 1H-NMR : δ = 2.65 (s, 3H, CH3), 7.18 –7.99 (m, 10H, Ar-H), 8.16 (s, 1H, CH), 9.17 and 14.14 (s, 2H, NH). M.F. C20H16ClN7O. Anal./Calcd: C(59.19); H(3.97); Cl(8.73); N(24.17); O(3.94). Found: C(59.13); H(3.94); Cl(8.75); N(24.12); O(3.94).
(E)‐1‐{N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]hydrazinecarbonyl}‐N‐(4‐chlorophenyl)methane-carbohydrazonoyl cyanide (12b)
m.p.: 248-250°C, yield: 0.35 g (81%), yellow (benzene). IR: 1666 (CO), 2220 (CN), 3035, 3256 (NH). M.F. C20H15Cl2N7O. Anal./Calcd: C(54.56); H(3.43); Cl(16.10); N(22.28); O(3.63). Found: C(54.59); H(3.37); Cl(16.12); N(22.30); O(3.61).
Synthesis of (2E)‐N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐2‐cyano‐3‐(dimethylamino)prop‐2‐enehydrazide (13)
N,N-dimethylformamide dimethyl acetal (0.001 mol) was added to (0.001 mol) of pyrazole derivative (2), in xylene (15 ml). The solution reaction heated for 4h at 220°C. The reaction solution was cooled, and the desired product was collected then dried. m.p.: 230-232°C, yield: 0.32 g (89%), pale yellow (1,4-dioaxne). IR: 1674 (CO), 2181 (CN), 3340 (NH). 1H-NMR: δ = 2.61 (s, 3H, CH3), 3.39 (s, 3H, CH3), 3.41 (s, 3H, CH3), 7.23–7.93 (m, 6H, Ar-H and CH ), 8.94 (s, 1H, CH) , 10.00 (s, 1H, NH ). M.F. C17H17ClN6O. Anal./Calcd: C(57.23); H(4.80); Cl(9.94); N(23.55); O(4.48). Found: C(57.16); H(4.77); Cl(9.90); N(23.60); O(4.43).
Preparation of 3‐amino‐N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐1H‐pyrazole‐4‐carbohydrazide (15)
(0.01l) of compound (13) was heated with alcoholic hydrazine (0.001 mol) in (15 ml) of 1,4-dioxane media. The reaction was continued heating for additional 7 h. The compound was solidified, then filtered, purified by ethanol,. m.p.: 105-106°C, yield: 0.28 g (87%) white (ethanol). IR: 1597, 1633 (C=N), 3208, 3364 (NH2). 1H-NMR: δ = 2.56 (s, 3H, CH3), 5.40 (s, 2H, NH2) and 7.19 – 7.51 (m, 7H, Ar-H), 7.67 (s, 1H, CH) and 8.59 (s, 2H, NH). M.F. C15H14ClN7O. Anal./Calcd: C(52.39); H(4.08); Cl(10,34); N(28.53); O(4.66). Found: C(52.35); H(4.10); Cl(10.31); N(28.54); O(4.62).
General Procedure for prepare N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐2‐cyano‐2‐[(2Z)‐4‐oxo‐3‐phenyl‐1,3‐thiazolidin‐2‐ylidene]acetohydrazide (22)
A (0.01 mol) of pyrazole derivative (2) was stirred with KOH (0.01 mol) in dry DMF (10 ml) in DMF followed by addition of phenyl isothiocyanate (0.01 mol)..The reaction was continued for additional 5h. under same condition Ethyl bromoacetate (0.01mol) was added with continues stirring at25°C for extra 4h. The reaction solution was triturated with diluted HCl (0.1 N). final product was solidified and separation. m.p.: 198-199°C, yield: 0.4 g (84%), red (ethanol). IR: 1734 (CO), 2201 (CN), 3254 (NH). 1H-NMR: δ = 2.58 (s , 3H, CH3 ), 4.15 (s, 2H, CH2), 7.25 – 7.55 (m, 10H, Ar-H), 8.04 (s, 1H, CH), 9.97 (s, 1H, NH). M.F. C23H17ClN6O2S. Anal./Calcd: C(57.93); H(3.59); Cl(7.43); N(17.62); O(6.71); S(6.72). Found: C(57.96); H(3.54); Cl(7.41); N(17.64); O(6.70); S(6.71).
Inhibition Potency of Microorganism
The synthesized compounds were tested against the selected microorganisms using. The standardized disc–agar diffusion method in[79,80]
Utilized Microorganisms
The microorganisms were used in the antimicrobial activity as represent in supporting information.
Results and Discussion
Chemistry
The starting material cyanoacetohydrazide derivative (2) was prepared via the reaction of ‐carbaldehyde derivative (1) with 2-cyanoacetohydrazide (Scheme 1). This starting material was exhibited prominent peak at 2263 cm-1 assignedfor (CN) at IR spectra. Where as the 1H-NMR (CDCl3) show signals at δ = 3.85 ppm characteristics for -CH2– and at 9.60 ppm for NH. when Treatment of N’‐[(1E)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐2‐cyanoacetohydrazide (2) with acetyl acetone was formed the corresponding pyrazolo-pyridone (4). The 1H-NMR showed signals (δ = 2.43 ppm ) characteristics for two Me groups and at 6.09 ppm stands for CH in pyridine.
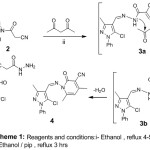 |
Scheme 1: Reagents and conditions: i- Ethanol, reflux 4-5 hrs ii-Ethanol/pip, reflux 3 hrs. |
However, reaction of (2) with salicaldehyde or its derivatives was afforded the corresponding N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐2‐imino‐6‐substituted chromene‐3‐carbohydrazide (6) via the plausible intermediate (5) as shown in (Scheme 2). The disappearance of absorption characteristics band for CN group at 2263 cm-1 in 2, and appearance of characteristics for NH at 3296 cm-1, that supported structure (6a). The other evidence supported (6a), disappearance of characteristic -CH2-signal (3.85 ppm), and presence of a characteristic signal for chromene-NH proton at 13.49 ppm. The 1H-NMR of (6b) exhibited a characteristic signal for CH3 of methoxy at 3.89 ppm.
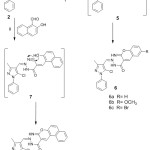 |
Scheme 2: Reagents and conditions: i-Ethanol/pip, reflux 2-3 hrs ii-Ethanol/pip, reflux 3 hrs. |
Moreover, coupling of (2) with 1-azido-4-fluorobenzene in presence of EtONa, which yielded the triazole derivative (10). The formation of triazole derivative (10) was assumed via the acyclic intermediate (9), which then cyclized via addition of NH2 to a CN groups (Scheme 2). The disappearance of an absorption band for a characteristic for CN group at 2263 cm-1 atinfrared (IR) spectrum, in addition to appearance of absorption band characteristics for NH2 at 3422 and 4552 cm-1. 1H-NMR showed the disappearance of signal at 3.85 ppm characteristic for -CH2– with presence of a characteristic band at 6.59 ppm for NH2 fragment, which supported compound (10).
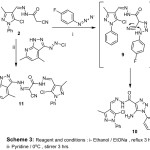 |
Scheme 3: Reagents and conditions: i-Ethanol/EtONa, reflux 2 hrs ii-Pyridine/0°C, stirrer 3 hrs. |
As well as, reaction of 3‐[(E)‐2‐chlorodiazen‐1‐yl]‐4,6‐dimethyl‐1H‐pyrazolo[3,4‐b]pyridine in pyridine afforded (11) via elimination of hydrogen chloride. At 1H-NMR , the compound (11) revealed the disappearance of characteristic for -CH2 signal at 3.85 ppm with appearance of characteristic new -NH signal at 11.03 ppm.
Also, reaction of benzenediazonium chloride derivative gave (12a, 12b),(Scheme 3). IR spectrum exhibited a prominent peak at 3256 cm-1 for (NH).1H-NMR exhibited the disappearance of signal at 3.85 ppm characteristic for -CH2– with appearance of signal at 14.14 ppm characteristic for the new NH. Reaction of pyrazole derivative (2) with N,N-Dimethylformamide dimethyl acetal in xylene was afforded substituted acrylohydrazide (13) via elimination of two methanol molecules. 1H-NMR data for compound (13) revealed the disappearance of peak at 3.85 ppm characteristic of -CH2– with absorption signal at 7.23 ppm characteristic for CH.
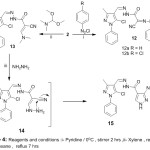 |
Scheme 4: Reagents and conditions: i- Pyridine/0°C, stirrer 2 hrs ii-Xylene, reflux 4 hrs, iii-1,4-Dioxane, reflux 7 hrs. |
The reaction of (13) with hydrazine hydrate in 1,4-dioxane resulted in formation of 3‐amino‐N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]‐1H‐pyrazole‐4‐carbohydrazide (15) via elimination of dimethylamine, then a cycloaddition reaction, which led to final product, as shown on (Scheme 4). The infrared (IR) spectrum showed presence of characteristics for NH2 bands at 3208, 3364 cm-1.
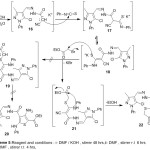 |
Scheme 5: Reagents and conditions: i- DMF/KOH, stirrer 48 hrs ii-DMF, stirrer r.t 6 hrs, iii-DMF,DMF, stirrer r.t 4 hrs. |
Compound (2) reacted with PhNCS in basic media to afford an intermediate product of potassium {N’‐[(1Z)‐(5‐chloro‐3‐methyl‐1‐phenylpyrazol‐4‐yl)methylidene]-hydrazinecarbonyl}(cyano)methanide (16), which, reacted with ethyl bromoacetate at room temperature to give phenylthiazol-2(3H)-ylideneacetohydrazide derivative (22) as showed in (Scheme 5). It can concluded that from the obtained spectral data, an elimination of hydrogen bromide and ethyl alcohol the most plausible mechanistic pathway for prepared compound (22) preferred over compound (20). the proposed mechanism was supported by intense absorption nitrile group band at 2201 cm-1 in IR spectrum, which would be disappeared of compound (20) formed. Whereas the 1HNMR spectrum showed no peaks for the characteristics signals of ethyl protons.
Table 1: Antimicrobial activity of the synthesized compounds.
| Mean* of zone diameter, nearest whole mm. | |||||||||||||
| Organisms | Gram – positive bacteria | Gram – negative bacteria | Yeasts and Fungi** | ||||||||||
| Staphylococcus aureus (ATCC 25923) | Bacillus subtilis (ATCC 6635) | Salmonella typhimurium (ATCC 14028) | Escherichia coli (ATCC 25922) | Candida albicans (ATCC 10231) | Aspergillus fumigatus | ||||||||
| Concentration Compounds | 1 mg/ml | 0.5 mg/ml | 1 mg/ml | 0.5 mg/ml | 1 mg/ml | 0.5 mg/ml | 1 mg/ml | 0.5 mg/ml | 1 mg/ml | 0.5 mg/ml | 1 mg/ml | 0.5 mg/ml | |
| 2 | _ | _ | _ | _ | 16 | 13 | _ | _ | 12 | 8 | _ | _ | |
| 3 | _ | _ | _ | _ | 14 | 12 | _ | _ | 9 | 7 | 21 | 17 | |
| 6 a | 11 | 8 | _ | _ | 14 | 13 | _ | _ | _ | _ | _ | _ | |
| 6 b | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | |
| 6 c | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | |
| 8 | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | |
| 12 a | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | |
| 12 b | _ | _ | _ | _ | _ | _ | _ | _ | 14 | 13 | _ | _ | |
| 13 | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | |
| 15 | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | |
| 22 | _ | _ | _ | _ | _ | _ | _ | _ | 13 | 10 | _ | _ | |
| Control # | 35 | 26 | 35 | 25 | 36 | 28 | 38 | 27 | 35 | 28 | 37 | 26 | |
* ,**, –, and #: = supporting information.
Biological Activity
Agar diffusion method used for evolution antimicrobial potency for tested compounds, using the following microorganism: “Gram- positive bacteria: Staphylococcus aureus (ATCC25923) and Bacillus subtilis (ATCC6635), Gram – negative bacteria: Escherichia coli (ATCC 25922) and Salmonella typhimurium (ATCC 14028), Yeast: Candida albicans (ATCC 10231) and Fungus: Aspergillus fumigatus”. From table 1, the compounds 6b, 6c, 8, 12a, 13 and were found to be inactive against all microorganisms while compounds 2, 3, 6a, 12b and 22 exhibited moderate activity against some microorganisms only and inactive against others. From the study, of the biological activity of these compounds it was found that these compounds containing the unit of pyrazole weak or no effect on microbes.
Conclusion
In this work, we prepared pyrazolo-cyanoacetohydrazide derivative (2) as a new stating material Pyrazolo-pyridone (4), pyrazolo-cheomene (6) and pyrazolo-benzochromene (8) was synthesized via nucleophilic cycloaddition reactions. when substituted aromatic azide reacted with (2), to give 5-aminotriazole through dipolar cycloaddition reaction mechanism. While, substitution reaction of (2) with diazonium chloride salts afforded (11) and (12). The compound (2) was reacted with phenylisothiocyanate in base medium followed by addition of ethyl bromoacetate afforded the pyrazolothiazole derivative (22). Antimicrobial activity was evaluated for all Synthesized compounds the compounds 6b, 6c, 8, 12a, 13 were exhibited inactive potency against all microorganisms while compounds 2, 3, 6a, 12b and 22 were exhibited moderate activity against some microorganisms Finally, we concluded that, the newly synthesized compounds possess a weak or no effect on microorganisms.
Acknowledgments
The author would like to thank everyone who helped in the success of this work.
Conflict of Interest
There is no conflict of interest.
References
- Hassan, A.; Ahmed, M.; Ali, Khalaf. Eur. J. Chem., 2014, 277-286.
- Luiza, R.; Raquel, R. Pharmaceuticals., 2012, 317-324.
- Lamberth, C.; Dinges, J. Wiley-VCH Verlag GmBH & Co. KGaA: Weinheim, Germany., 2012, 175–194.
- Elguero, J.; Goya, P.; Jagerovic, N.; Silva, A.M. Ital. Soc. Chem., 2002, 52‐98.
- Robin, E. M. ; David, R.G.; Vijayalekshmi. S. Phytochemistry., 2008, 69, 2704‐2707.
CrossRef - Karthikeyan, M. S.; Holla, B.S.; Kumari, N. S. Eur. J. Med. Chem., 2007, 42, 30‐36.
CrossRef - Bandgar, B. P.; Totre, J. V.; Gawande, S. S.; Khobragade, C. N.; Warangkar, S. C.; Kadam, P. D. Bioorg. Med. Chem., 2010, 18(16) , 6149-6155 .
CrossRef - Prakash, O.; Rashmi, P.; Pooja, R.; Kamaljeet, P.; Yogita, D.; Aneja, K. R., Indian J Chem., 2009 , 48B , 563-568.
- Bekhit, A. A.; Ashour, H. M.; Guemei, A. A. Arch Pharm (Weinheim)., 2005, 338(4) , 167-174.
CrossRef - Xia, Y.; Fan,C.; Zhao, B.X.; Zhao, J.; Shin, D. S.; Miao, J.Y. Eur. J. Med. Chem., 2008, 43, 2347‐2353.
CrossRef - Brahmbhatt, D. I.; Ankit, R. K.; Anil, K. P.; Niraj, H. P. Indian. J. Chem., 2010, 49B , 971-977.
- Michael, J. G.; Carolyn, B. J. Med. Chem., 2000, 43(5) ,1034-1040.
- Zhang, A.; Zhou, J.; Tao, K.; Hou, T.; Jin, H. Bioorg. Med. Chem. Lett., 2018, 28, 3042-3045.
CrossRef - Vala, ND.; Jardosh, HH.; Patel, MP. Chin. Chem. Lett., 2016, 27(1), 168-172.
CrossRef - Parmar, BD.; Sutariya, TR.; Brahmbhatt, GC.; Parmar, NJ.; Kant, R.; Gupta, VK.; Murumkar, PR.; Sharma, MK.; Yadav, MR. Molecular. diversity., 2016, 20(3), 639-657.
CrossRef - Mondal, G.; Jana, H.; Acharjya, M.; Santra, A.; Bera, P.; Jana, A.; Panja, A.; Bera, P. Med. Chem. Res., 2017, 26(11), 3046-3056.
CrossRef - Atta-Allah, SR.; Abou-Elmagd, WS.; Kandeel, KA.; Hemdan, MM.; Haneen, DS.; Youssef, Ahmed, S. J. Chem. Res., 2017, 41(11), 617-623.
CrossRef - El-Gamel, NE.; Farghaly, TA. Spectr. Acta., 2013, 115, 469-475.
CrossRef - Bondock, S.; Fadaly, W.; Metwally, MA. Eur. J. Med. Chem., 2010, 45(9), 3692-3701.
CrossRef - Imran, M.; Bakht, MA.; Samad, A.; Abida A. Tropical. J. Pharm. Res., 2017, 16(5), 1147-1155.
CrossRef - Ansari, MI.; Khan, SA. Med. Chem. Res., 2017, 26(7), 1481-1496.
CrossRef - Khidre, RE.; Abu-Hashem, AA.; El-Shazly, M. Eur. J. Med. Chem., 2011, 46(10), 5057-5064.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










