Nanocrystalline Titania Coated Metakaolin and Rice Hull Ash Based Geopolymer Spheres for Photocatalytic Degradation of Dye in Wastewater
Patricia Isabel Bravo1, Eiza Shimizu1, Roy Alvin Malenab2,April Anne Tigue2, Kimmie Mae Dela Cerna2, Jose Isagani Janairo3,4, Michael Angelo Promentilla2,4 and Derrick Ethelbhert Yu*1,4
1Department of Chemistry, De La Salle University, 2401 Taft Avenue, Manila, Philippines.
2Department of Chemical Engineering, De La Salle University, 2401 Taft Avenue, Manila, Philippines.
3Department of Biology, De La Salle University, 2401 Taft Avenue, Manila, Philippines.
4Materials Science and Nanotechnology Unit, De La Salle University, 2401 Taft Avenue, Manila, Philippines.
Corresponding Author E-mail: derrick.yu@dlsu.edu.ph
DOI : http://dx.doi.org/10.13005/ojc/350118
Article Received on : 18-01-2019
Article Accepted on : 05-02-2019
Article Published : 25 Feb 2019
Geopolymer spheres made from metakaolin and rice hull ash have exceptional chemical and mechanical stability, making them promising catalyst support matrix for photoactive compounds. In this paper, titania was deposited on geopolymer sphere via horizontal vapor phase growth method, which produced nanotitania crystals embedded on the surface. The titania-coated geopolymer spheres resulted in 90% dye photodegradation activity that can be used repeatedly, thus qualifying the products as sustainable materials for wastewater treatment.
KEYWORDS:Degradation; Geopolymer; Nanotitania Photocatalyst; Photocatalytic Nanotitania Deposition
Download this article as:| Copy the following to cite this article: Bravo P. I, Shimizu E, Malenab R. A, Tigue A. A, Cerna K. M. D, Janairo J. I, Promentilla M. A, Yu D. E. Nanocrystalline Titania Coated Metakaolin and Rice Hull Ash Based Geopolymer Spheres for Photocatalytic Degradation of Dye in Wastewater. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Bravo P. I, Shimizu E, Malenab R. A, Tigue A. A, Cerna K. M. D, Janairo J. I, Promentilla M. A, Yu D. E. Nanocrystalline Titania Coated Metakaolin and Rice Hull Ash Based Geopolymer Spheres for Photocatalytic Degradation of Dye in Wastewater. Orient J Chem 2019;35(1). Available from: https://bit.ly/2EvOxUr |
Introduction
The increasing variety and amount of hazardous compounds produced and discharged by the printing, dyeing, textile and various chemical industries mainly contribute to water pollution. Advanced oxidation processes (AOPs) by generation of reactive oxygen species (ROS) are proven to be most effective in wastewater treatment, due to the high oxidative power of radicals that produce stable, more biodegradable by-products.1 Among the photocatalysts used in AOPs, titanium dioxide or more commonly known as titania (TiO2), is the most efficient and the most widely used photocatalyst for photodegradation of organic and inorganic dyes in wastewaters. It exists in a variety of polymorphs, such as anatase, rutile and brookite, with Eg = 3.2, 3.0 and 2.2 eV, respectively, giving different photocatalytic activities. Aside from the crystal orientation, the photocatalytic activity of titania is also influenced by its particle size and morphology. Reduction of particle size to nanoscale increases its photoactivity due to the added discrete energy levels formed as a result of confinement effects,2 while extension of surface area from spheres to rods/belts would have lower recombination rate due to increased charge mobility along the longitudinal dimension.3 Currently, titania is used in wastewater treatment either by suspension or immobilization.2 However, immobilization of the photocatalyst is more practical and preferred since it allows easy collection and recycling of titania4. Several deposition techniques employ glass as the substrate, however, the aim of producing a reusable material is limited by the brittle property of glass. A substitute to glass substrate is geopolymer.
Geopolymers (GPs) are inorganic polymers formed by the alkaline activation of alumino-silicate materials having tetrahedral units of varying Al/Si ratio, which may come from different industrial wastes such as metakaolin, fly ash and rice husk ash5. It has the potential to replace ordinary Portland cement due to its less carbon footprint, enhanced mechanical properties, and environment-friendliness.6 Its formation involves deconstruction of the Al-Si content in the precursor due to reaction with the alkaline activator, followed by polymerization and polycondensation, forming the highly stable, semicrystalline three-dimensional cross-linked Al-Si-O species.7 GPs are stable up to 1300°C6 and resistant to wastewater photocatalytic degradation by-products,8 making it a suitable support matrix for titania deposition. TiO2 is deposited on the geopolymer sphere surface using Horizontal Vapor Growth Deposition (HVPG) technique. HVPG technique has been known to produce nanocrystalline semiconductors, including organic semiconductors, because it permits milder experimental conditions that preserves the integrity of the chemical structure.9 Thus, TiO2 deposited on GP surface by HVPG technique will make an environment-friendly material that is expected to have excellent photoactivity.
The goal of this study is to make a promising and economical photocatalyst material by depositing nanotitania on a GP sphere by horizontal vapor phase growth (HVPG) technique, for dye degradation in wastewater systems. The GP sphere uses MK and RHA waste materials, which serves as an eco-friendly substrate for nanotitania synthesis. HVPG technique permits nanostructured TiO2 deposition on the surface, to produce an efficient, economical material. Morphological characterization is evaluated using Scanning Electron Microscope (SEM), chemical characterization by Fourier Transform Infrared Spectroscopy (FT-IR), and elemental composition by Energy Dispersive X-Ray (EDX). The photocatalytic activity is determined in terms of methylene blue degradation, which was evaluated by UV-Vis Spectroscopy.
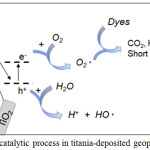 |
Scheme 1: Photocatalytic process in titania-deposited geopolymer sphere. |
Materials and Methods
Metakaolin (MK) and rice hull ash (RHA) were used and received from a materials recovery facility in Tarlac Province, Philippines. Sodium hydroxide (NaOH), commercial water glass solution (WGScommercial, modulus = 2.5; 34.1 wt % SiO2, 14.7% Na2O), polyethylene glycol with average molecular weight of 600 g/mol (PEG-600), polysorbate 80 and titanium (IV) oxide anatase (99.8%) were used without further processing or purification.
Synthesis of Geopolymer Spheres
The synthesis of geopolymer spheres was done through modified procedures.10 RHA (16 g, 192 mmol SiO2) and NaOH (5.33g, 133 mmol) were suspended in 32mL water, and was refluxed for 4 hours at 90°C. The filtered solution yielded clear homogenous water glass solution (modulus = 2.8). Then, MK (16 g, 145 mmol SiO2, 68.6 mmol Al2O3), polysorbate 80 (0.3 g) and distilled H2O (4.6 g, 256 mmol) were added to 6.5 g prepared water glass solution (WGSprep), forming an orange-brown slurry. The slurry was transferred in a 10mL syringe and was dropped into PEG – 600 solution at 60°C. Collected spheres were washed with acetone and cured at 75°C for 2 days, producing brown spheres with diameter 2-3 mm.
Titania Deposition on Geopolymer Sphere by HVPG
Figure 1 depicts the set-up for the deposition of TiO2 on the geopolymer sphere. GP spheres, glass wool and TiO2 powder (10 mg, 0.125 mmol) were placed in a closed-end quartz tube and sealed to give an evacuated tube (pressure = 5×10-6 Torr) that is sealed at both ends. An oxygen fuel torch was used to seal the tubes, while Thermionics Vacuum System was used as vacuum. The sealed tube is horizontally placed in a conventional furnace, with the spheres end protruding outside, and was baked at 1200°C for 6 hours.
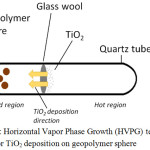 |
Figure 1: Horizontal Vapor Phase Growth (HVPG) technique set-up for TiO2 deposition on geopolymer sphere. |
Table 1: Percent chemical composition of Metakaolin and Rice Husk Ash sourced in Tarlac Province determined by X-Ray Fluorescence (XRF) Spectroscopy.
| Oxides | Metakaolin | Rice hull ash |
| SiO2 | 54.5 | 72.2 |
| Al2O3 | 43.7 | – |
| Fe2O3 | 0.8 | 8.2 |
| K2O | 0.5 | 8.5 |
| CaO | 0.2 | 7.1 |
| others | 0.4 | 4.0 |
| LOI | 0.6 | 12.1 |
Materials Characterization
The IR profile of TiO2-deposited GP sphere was analyzed using FT-IR spectrophotometer (Nicolet Magna-IR 550) in KBr disk, while morphological characterization and elemental analysis were evaluated using SEM-EDX (Phenom XL 2015 LR1).
Photocatalysis Experiment
The photocatalytic experiment was carried out in triplicate using a self-made photoreactor. Initially, TiO2-deposited GP spheres were suspended in methylene blue solution in a sealed glassware, placed in a mixer and was allowed to stir for 30 minutes in the dark, to establish an absorption/desorption equilibrium. Then, the solution was illuminated with UV light for 10 hours. The UV light bulb is approximately 12 inches above the reaction mixture. The concentration of the final solution was determined by measuring its absorbance using UV-Vis spectrophotometer (Hitachi U-2900). The % degradation was calculated as described in Equation 1.
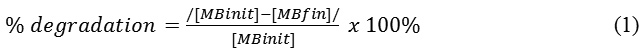
where [MBinit] = initial MB concentration.
[MBfin] = MB concentration after UV exposure.
Results and Discussion
GP Sphere Synthesis
With the aim of providing practical materials with green chemistry, RHA and MK were used as raw materials for the synthesis of geopolymer spheres. As shown in Table 1, the 72.2% SiO2 content in RHA make it an ideal source of silica for the synthesis of WGS, which serves as the alkaline activator of MK. The produced geopolymer spheres had nonuniform porous structure (Figure 2a), where no degradation and morphological changes were observed even after the deposition procedure (Figure 2b), which makes it a suitable green substrate material for the deposition of titania.
Nanostructure Formation
HVPG technique is a method capable of converting TiO2 powder into nanostructures. Vapor deposition is done in a vacuum sealed quartz or silica tube, and because of the low pressure inside the tube, even nonvolatile compounds are possible to vaporize in moderate temperatures. The set up inside the quartz tube for the TiO2 deposition on the GP sphere is shown in Figure 1. Initially, the GP spheres and anatase TiO2 were separated using a glass wool to hold titania in place, away from the GP spheres. Upon baking, sublimed TiO2 particles travelling towards the GP spheres in the cooler region of the tube deposit on the GP surface. The dwelling time and temperature were varied to determine the optimal conditions for the nanotitania deposition. Results showed that nanotitania deposition is optimal at baking temperature 1200°C and 6 hours dwelling time, which produced nanorod morphology that is approximately 200 nm wide and 1-3μm long (Figure 3).
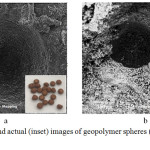 |
Figure 2: SEM and actual (inset) images of geopolymer spheres (a) before and (b) after deposition. |
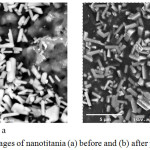 |
Figure 3: SEM images of nanotitania (a) before and (b) after photocatalysis. |
The deposition of TiO2 on the geopolymer surface was further confirmed by elemental map images, which shows the presence of silicon, aluminum and titanium, as shown in Figure 4. This was further confirmed by EDX elemental analyses, which shows approximately 2:2:1 weight percent ratio for Si:Al:Ti.
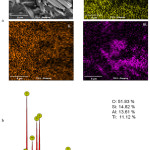 |
Figure 4: (a) SEM image with (i) silicon, (ii) aluminum and (iii) titanium elemental map and (b) EDX data of TiO2-GP. |
Structure of TiO2-deposited GP Sphere
The microstructure of a geopolymer matrix is composed of geopolymeric nanoparticles / micelles that gives its overall characteristic features, such as having excellent chemical and mechanical stability.5,6,11 These GP micelles initially have OH groups on the surface,6 as illustrated on Figure 5a, which then undergoes condensation reaction with TiO2 during HVPG process that forms a covalently linked TiO2-GP material through the formation of Ti-O-Si bond. This is confirmed by FTIR analysis of the TiO2-GP material, as shown in Figure 6, and is compared to the GP and anatase TiO2 spectra. The bands at ~1100 and 840 cm-1 in GP are assigned to Si-O-Si and Si-O-Al asymmetric stretching vibrations respectively,11,12 where in the TiO2-GP spectra, the 840 cm-1 band is overlapped in the broad 500 – 800 cm-1 peak that is assigned to Ti-O-Ti band. The 3460 and 1641 cm-1 band in GP, which is assigned to -OH and H-O-H stretching and bending vibrations,12,13 indicates the presence of adsorbed water on the GP surface. In TiO2-GP, the intensity of these peaks (1) are significantly reduced due to decreased Si-OH species, which signifies the formation of Si-O-Ti on the GP surface, and (2) are displaced towards lower wavenumber (3412 and 1632 cm-1) due to formation of Ti-O-H species. Furthermore, the absence of 2900, 1460 and 1352 cm-1 bands in the TiO2-GP spectra indicate elimination of the carbon groups initially present in the GP sphere. These peaks are assigned to the C-H vibrations of unreacted NaOH carbonated by atmospheric CO2, which are then eliminated by the high temperature conditions in HVPG technique.
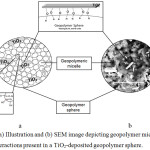 |
Figure 5: (a) Illustration and (b) SEM image depicting geopolymer microstructure and the interactions present in a TiO2-deposited geopolymer sphere. |
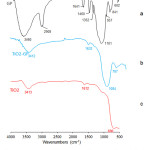 |
Figure 6: FTIR Spectra of (a) geopolymer, (b) Anatase TiO2, and (c) TiO2 deposited on GP matrix. |
Photocatalytic Activity Evaluation
The photocatalytic activity of the synthesized TiO2-GP spheres was evaluated in terms of methylene blue degradation, where results reveal that after 10 hours exposure, approximately 93% methylene blue degradation was observed. This is due to the embedded nanobelt morphology of TiO2 on the GP surface provides more redox zones and greater charge mobility, resulting in reduced recombination rate, and consequently enhanced titania photoactivity. After the photocatalysis experiment, the spheres were then subjected to SEM, elemental mapping and 3D image analyses, to check for any morphological and chemical changes. As shown in Figure 7, SEM and elemental mapping images reveal intact TiO2 on the GP sphere surface. Further, 3D imaging analysis of a TiO2-GP sphere region showed TiO2 nanobelts that are embedded on the GP surface (Figure 7c). This suggests that even after photocatalysis, no significant morphological changes were observed, thus, TiO2-GP sphere may be recycled. To check this hypothesis, the same set of TiO2-GP spheres were again tested for photocatalytic activity, employing the same conditions. The absorption spectra of methylene blue before and after exposures is shown in Figure 8, revealing similar methylene blue degradation (approximately 91%) even after second exposure.
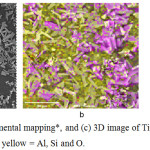 |
Figure 7: (a) SEM, (b) elemental mapping*, and (c) 3D image of TiO2-GP after photocatalysis. *pink = Ti; yellow = Al, Si and O. |
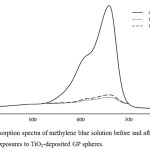 |
Figure 8: Absorption spectra of methylene blue solution before and after the first and second exposures to TiO2-deposited GP spheres. |
Conclusion
The geopolymer sphere synthesized from recycled materials (i.e. metakaolin, which served as the aluminosilicate source, and rice hull ash, as the silicate source of the activating solution) were shown to be promising catalyst support matrix for nanotitania deposition. Titania was deposited via a modified horizontal vapor phase growth technique, producing an efficient photocatalyst for pollutant degradation. Approximately 90% degradation was reported, which was shown to give similar results when the geopolymer photocatalysts were reused. Thus, the synthesis of TiO2-deposited geopolymer sphere is an efficient, economical and sustainable material that may be utilized in photocatalytic degradation of dye pollutants in wastewater.
Acknowledgements
The authors are grateful for the financial support from the University Research Coordination Office (URCO) of De La Salle University.
References
- Deng, Y.; Renzun, Z. Curr. Pollut. Reports, 2015, 1, 167–176.
- Jain, A.; Vaya, D. J. Chil. Chem. Soc., 2017, 62, 3683–3690.
- Wu, N.; Wang, J.; De Nyago, T.; Wang, H.; Zheng, J-G.; Lewis, J.P.; Liu, X.; Leonard, S.S.; Manivannan, A. J. Am. Chem. Soc., 2010, 132, 6679–6685.
CrossRef - Lazar, M.A.; Varghese, S.; Nair, S.S. Catalysts, 2012, 2, 572–601.
CrossRef - Davidovits, J. J. Therm. Anal., 1991, 37, 1633–1656.
- Davidovits, J. J. Ceram. Sci. Technol., 2017, 8, 335–350.
- Yao, X.; Zhang, Z.; Zhu, H.; Chen, Y. Thermochim. Acta, 2009, 493, 49–54.
CrossRef - Bakharev, T. Cem. Concr. Res., 2005, 35, 658–670.
- Shimizu, E.; Santos, G.N.; Yu, D.E. J. Nanotechnol., 2016, 5175462.
- Tang, Q.; Ge, Y.; Wang, K.; He, Y.; Cui, X. J. Mater. Des., 2015, 88, 1244–1249.
- Schmucker, M.; MacKenzie, K.J.D. Ceram. Int., 2005, 31, 433–437.
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Int. J. Inorg. Mater., 2000, 2, 309–317.
CrossRef - Pinho, L.; Mosquera, M.J. J. Phys. Chem. C, 2011, 115, 22851–22862.

This work is licensed under a Creative Commons Attribution 4.0 International License.









