Extraction of Non-Ferrous Metals as Inorganic Chlorides from Waste Lead Slags in the Presence of Chlorine-Containing Components of a Distilled Liquid
Dana Temirbekovna Pazylova* , Viktor Mikhaylovich Shevko, Alibek Spabekovich Tleuov, Nurila Saidullaevna Saidullayeva, Asiya Salidinovna Abzhanova and Nurzhamal Mahamatovna Ablyazimova
, Viktor Mikhaylovich Shevko, Alibek Spabekovich Tleuov, Nurila Saidullaevna Saidullayeva, Asiya Salidinovna Abzhanova and Nurzhamal Mahamatovna Ablyazimova
South Kazakhstan State University named after M. Auezov, 160012, Tauke Khan avenue 5., Shymkent, Kazakhstan.
Corresponding Author E-mail: danapazyl@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/350152
Article Received on : 08-12-2018
Article Accepted on : 26-01-2019
Article Published : 20 Feb 2019
The presented article contains the research results on extraction of non-ferrous metals as inorganic chlorides from waste lead slags in the presence of chlorine-containing components of a distilled liquid – calcium and sodium chlorides. The research was implemented by a thermodynamic modelling technique using a software package HSC-5.11 Chemistry, based on a Gibbs energy minimum principle, and also a rototable second-degree method of planning an experiment (Box-Hunter method). It was found, that at equilibrium conditions lead is extracted from slags by chloride sublimation the most effectively, then zinc and copper: at pressure of 1 bar and temperature of 1500°С the chloride sublimation degree for lead makes 98.63%, for zinc – 64.45% and for copper – 21.91%. To achieve the 95-100% lead chloride sublimation degree the necessary temperature is 933.7‒1300°С and lgP should be from –0.48 to –1.75 (pressure of 0.33-0.0177 bar); for achievement of the 90.0-100% chloride sublimation degree for zinc respective parameters are T=1279.3–1500°C and lgP from –0.972 to –2 (pressure of 0.107-0,01 bar), for copper ‒ temperature of 1337.7–1500°С and lgP from –1.418 to –2 (pressure of 0.038-0.01 bar). It was experimentally proved, that at the sinter-chlorinating roasting at temperature of 1150-1180°С and pressure of 0.006-0.001 bar the chloride sublimation degree for lead makes 93-98%, for zinc ‒ 82-96%, for copper – 73-88%.
KEYWORDS:Chloride Sublimation of Lead; Distilled Liquid; Fuming Slags; Sinter-Chlorinating Roasting; Sodium and Calcium Chlorides; Thermodynamic Modelling; Zinc and Copper
Download this article as:| Copy the following to cite this article: Pazylova D. T, Shevko V. M, Tleuov A. S, Saidullayeva N. S, Abzhanova A. S, Ablyazimova N. M. Extraction of Non-Ferrous Metals as Inorganic Chlorides from Waste Lead Slags in the Presence of Chlorine-Containing Components of a Distilled Liquid. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Pazylova D. T, Shevko V. M, Tleuov A. S, Saidullayeva N. S, Abzhanova A. S, Ablyazimova N. M. Extraction of Non-Ferrous Metals as Inorganic Chlorides from Waste Lead Slags in the Presence of Chlorine-Containing Components of a Distilled Liquid. Orient J Chem 2019;35(1). Available from: https://bit.ly/2ScAMxt |
Introduction
The ammoniac way prevailing in the world soda production gives a considerable quantity of a liquid waste product – distilled liquid. Its composition, g/m3: СаСl2– 118-125; NaCl – 58.6-80; CaCO3– 6-15; CaSO4– 2-2.7; Mg(OH)2 – 3-10; CaO – 2-4; Fe2O3 +Al2O3 – 1-3; SiO2–1-4.1 There are some directions of partial recycling of the distilled liquid that allows obtaining: waterless calcium peroxide used for production of bleachers and disinfectants and for treatment of waste water and gas emissions2; phosphorus-containing fertilizers applied in agriculture3; ammonium chloride, which can be used in the textile industry, pharmaceutics, at soldering of metals, tinning, for filling of galvanic cells, as a nitric fertilizer4; high-quality chemically-precipitated calcium carbonate widely used in many industries for manufacturing various composite materials.5 After special preparation the distilled liquid can be applied for pumping in oil wells for the purpose of maintenance of strata pressure.6 But such the way of its recycling is possible only if soda ash manufacture is situated in the oil recovery area. There is a method of electrochemical processing of the distilled liquid with obtaining Са(OH)2, NaОН and НСl.7 Also the distilled liquid is used at soda ash manufacturing by means of processing of a salt solution for desulfation of a brine and production of gypsum.8 We suggest to use the distilled liquid for extraction of nonferrous metals as inorganic chlorides fromfuming slags.
Experimental Section
Materials and Methods
At implementation of the research we applied the fuming slags of JSC «Yuzhpolimetal» containing (%): ZnSiO3‒6.4; ZnS ‒1.5;Cu2O‒ 1.5; PbS‒0.2; PbSO4‒ 0.2;SiO2‒22.7; Fe2O3‒40.1; CaO‒13.9; MgO‒3.1; Al2O3‒5.4; MnO‒ 0.7; Na2O‒2.91; others – 1.4. A mixture of calcium and sodium chlorides with a mass ratio of CaCl2/NaCl=2(such the chloride ratio is typical of the distilled liquid. Its composition, g/m3: СаСl2– 118-125; NaCl – 58.6-80; CaCO3– 6-15; CaSO4– 2-2.7; Mg(OH)2 – 3-10; CaO – 2-4; Fe2O3 +Al2O3 – 1-3; SiO2– 1-4) was used as a chlorinating agent. The chlorides content was constant and equal 6.8% of CaCl2 and 3.4% of NaCl of the slag weight. The oxygen mass also was constant and made 100% of the theoretically necessary weight for oxidation of lead and zinc sulphides to their oxides.
The research was implemented by a thermodynamic modelling technique using a software package HSC-5.11 Chemistry, based on a Gibbs energy minimum principle,9 and also a rototable second-degree method of planning an experiment (Box-Hunter method).10 First of all, we studied the pressure and temperature effect on the quantitative distribution of substances in a system under study, and then on the basis of the obtained data–the equilibrium chloride sublimation degree (αchl) of lead, zinc and copper. The metals’ chloride sublimation degree was calculated according to the expression:
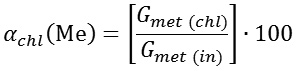
whereGmet (chl) ‒ weight of a metal turned into chloride, kg; Gmet (in)‒ weight of a metal in slag, kg.
Results and Discussion
Temperature and Pressure Effect on the Quantitative Distribution of the Substances Containing Lead, Zinc and Copper in a System Slag – (CaCl2 + NaCl) ‒ О2
Fig. 1 contains the information obtained by means of the software packageHSC-5.11 about the quantitative distribution of the substances containing lead, zinc and copper at pressure of 1 and 0,1 bar. As follows from the figure, lead in the studied system is as PbCl2, PbCl2(g), PbCl(g), PbSO4; zinc as ZnSiO3, ZnCl2(g), ZnCl2 and copper as Cu2O, CuCl, CuCl2(g), CuCl(g). Decrease in the pressure promotes increase in αchl(Me) and reduction of the process temperature.
 |
Figure 1: Temperature and pressure effect on the quantitative distribution of the substances containing lead, zinc and copper in a system slag – (CaCl2 + NaCl) ‒ О2. |
Temperature Effect on the Chloride Sublimation Degree of Lead, Zinc and Copper from the Slag in the Presence of CaCl2 and NaCl
Influence of temperature and pressure on αchl(Me)is represented in fig. 2.
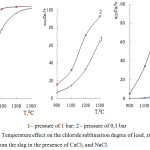 |
Figure 2: Temperature effect on the chloride sublimation degree of lead, zinc and copper from the slag in the presence of CaCl2 and NaCl. |
Judging from the fig. 2 the decrease in pressure and increase in temperature leads to increase in αchl(Me). We accept αchl(Pb) >αchl(Zn) >αchl(Cu).
The Research Matrix and Results
The research matrix (according to the second-degree rototableplanning) and results are represented in tables 1-3. On the basis of the results the regression equations of temperature (Т,°С) and pressure (lgP, bar) influence on the chloride sublimation degree of lead (αchl(Pb), %), zinc (αchl(Zn), %) and copper (αchl(Cu), %) were obtained:
αchl(Pb) = – 114.491–60 T,°C +0.2772 · T–5 · (lgP)2 T,°C 10-5 · T2 0.0367 · lgP T,°C
αchl(Zn) = – 622.1–101.8 · lgP +0.823 · T–7.72 · (lgP)2 – 2.44 · 10-4 · T2 +0.045 · lgP · T;
αchl(Cu) = – 1635.89 – 164.44 · lgP + 2.103 · T – 7.76 · (lgP)2 – 6.62 · 10-4 · T2+ 0.0735 · lgP · T.
Using these equations according to the technique11 solid surfaces of temperature and pressure effect on αchl(Pb),αchl(Zn) and αchl(Cu)and their horizontal sections were constructed (fig.3). As follows from fig. 3, αchl(Pb) from 95 to 100% is observed in the area abcd, αchl(Zn) from 90 to 100% ‒ in the area xyzf and αchl(Cu)‒ in the area nmet. Values of temperature and pressure in boundary points of these technological areas are represented in table 4.
Table 1: The planning matrix and the research results of the lead chloride sublimation from the fuming slag.
| № |
Variable parameter |
Lead chloride sublimation degree,% |
||||
|
Code kind |
Natural kind |
αchl (initial) |
αchl (calcul.) | |||
|
х1 |
х2 |
lgP, bar |
T,°C |
|||
| 1 | +1 | +1 | -0.3 (0.50) | 1241.8 |
95.01 |
93.63 |
| 2 | +1 | -1 | -0.3 (0.50) | 958.2 |
76.52 |
74.79 |
| 3 | -1 | +1 | -1.7 (0.02) | 1241.8 |
99.92 |
99.82 |
| 4 | -1 | -1 | -1.7 (0.02) | 958.2 |
96.03 |
95.58 |
| 5 | +1.414 | 0 | -0.0 (0.00) | 1100 |
79.04 |
80.80 |
| 6 | -1.414 | 0 | -2.0 (0.01) | 1100 |
99.91 |
99.87 |
| 7 | 0 | +1.414 | -1.0 (0.1) | 1300 |
99.12 |
99.74 |
| 8 | 0 | -1.414 | -1.0 (0.1) | 900 |
123.03 |
83.42 |
| 9 | 0 | 0 | -1.0 (0.1) | 1100 |
95.03 |
95.24 |
| 10 | 0 | 0 | -1.0 (0.1) | 1100 |
95.32 |
95.24 |
| 11 | 0 | 0 | -1.0 (0.1) | 1100 |
94.71 |
95.24 |
| 12 | 0 | 0 | -1.0 (0.1) | 1100 |
94.83 |
95.24 |
| 13 | 0 | 0 | -1.0 (0.1) | 1100 |
96.44 |
95.24 |
Table 2: The planning matrix and the research results ofthe zinc chloride sublimation from the fuming slag.
| № |
Variable parameter |
Zinc chloride sublimation degree,% |
||||
| Code kind |
Natural kind |
αchl (initial) |
αchl (calcul.) | |||
| х1 | х2 | lgP(bar) | T,°C | |||
| 1 | +1 | +1 | -0.3 (0.50) | 1441.8 |
70.02 |
68.48 |
| 2 | +1 | -1 | -0.3 (0.50) | 1158.2 |
22.21 |
18.72 |
| 3 | -1 | +1 | -1.7 (0.02) | 1441.8 |
65.33 |
66.51 |
| 4 | -1 | -1 | -1.7 (0.02) | 1158.2 |
33.33 |
36.68 |
| 5 | +1.414 | 0.0 | -0.0 (0.00) | 1300.0 |
95.01 |
91.61 |
| 6 | -1.414 | 0.0 | -2.0 (0.01) | 1300.0 |
92.02 |
90.75 |
| 7 | 0.0 | +1.414 | -1.0 (0.1) | 1500.0 |
31.84 |
33.04 |
| 8 | 0.0 | -1.414 | -1.0 (0.1) | 1100.0 |
71.01 |
71.72 |
| 9 | 0.0 | 0.0 | -1.0 (0.1) | 1300.0 |
71.02 |
71.72 |
| 10 | 0.0 | 0.0 | -1.0 (0.1) | 1300.0 |
72.33 |
71.72 |
| 11 | 0.0 | 0.0 | -1.0 (0.1) | 1300.0 |
73.12 |
71.72 |
| 12 | 0.0 | 0.0 | -1.0 (0.1) | 1300.0 |
71.63 |
71.72 |
| 13 | 0.0 | 0.0 | -1.0 (0.1) | 1300 |
71.71 |
71.72 |
Table 3: The planning matrix and the research results of the copper chloride sublimation from the fuming slag.
| № |
Variable parameter |
Copper chloride sublimation degree,% | ||||
| Code kind |
Natural kind |
αchl (initial) |
αchl (calcul.) | |||
| х1 | х2 | lgP(bar) | T,°C | |||
| 1 | +1 | +1 | -0.3 (0.50) | 1370.7 |
36.42 |
41.10 |
| 2 | +1 | -1 | -0.3 (0.50) | 1329.3 |
12.21 |
8.93 |
| 3 | -1 | +1 | -1.7 (0.02) | 1370.7 |
97.21 |
98.13 |
| 4 | -1 | -1 | -1.7 (0.02) | 1329.3 |
88.02 |
80.57 |
| 5 | +1.414 | 0.0 | -0.0 (0.00) | 1400.0 |
13.03 |
11.22 |
| 6 | -1.414 | 0.0 | -2.0 (0.01) | 1400.0 |
98.11 |
102.0 |
| 7 | 0.0 | +1.414 | -1.0 (0.1) | 1500.0 |
80.32 |
75.24 |
| 8 | 0.0 | -1.414 | -1.0 (0.1) | 1300.0 |
33.13 |
40.07 |
| 9 | 0.0 | 0.0 | -1.0 (0.1) | 1400.0 |
64.52 |
64.32 |
| 10 | 0.0 | 0.0 | -1.0 (0.1) | 1400.0 |
63.04 |
64.32 |
| 11 | 0.0 | 0.0 | -1.0 (0.1) | 1400.0 |
62.92 |
64.32 |
| 12 | 0.0 | 0.0 | -1.0 (0.1) | 1400.0 |
65.23 |
64.32 |
| 13 | 0.0 | 0.0 | -1.0 (0.1) | 1400.0 |
63.91 |
64.32 |
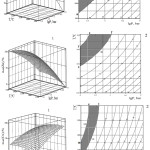 |
Figure 3: Temperature and pressure effect on the chloride sublimation degree of lead, zinc and copper in a system slag – (CaCl2 + NaCl) ‒ О2. |
Table 4: Technological parameters of the optimal areas of the metals’ chloride sublimation from the slags by the use of the calcium and sodium chlorides mixture typical for the distilled liquid.
| Metal (area, figure) |
Parameter |
Points |
||||
|
а |
b |
c |
d |
|||
|
Lead (abcd, fig.3, І) |
T,°C |
933.7 |
1130.1 |
1300 |
1300 |
|
|
lgP(bar) |
-1.75(0.0177) |
-1.75(0.0177) |
-1.195(0.06) |
-0.48(0.33) |
||
| αchl,% |
95.0 |
100.0 |
100.0 |
95.0 |
||
|
Zinc (xyzf, fig.3, ІІ) |
Parameter |
Points |
||||
|
x |
y |
z |
f |
|||
|
T,°C |
1279.3 |
1417.1 |
1500 |
1500 |
||
|
lgP(bar) |
-2.0(0.01) |
-2.0(0.01) |
-1,695(0.02) |
-0.972(0.1067) |
||
|
αchl,% |
90.0 |
100.0 |
100.0 |
90.0 |
||
|
Copper (nmet, fig.3, ІІІ) |
Parameter |
Points |
||||
|
n |
m |
e |
t |
|||
|
T,°C |
1337.7 |
1382.9 |
1500 |
1500 |
||
|
lgP(bar) |
-2.0(0.01) |
-2.0(0.01) |
-1.75(0.0177) |
-1.418(0,381) |
||
|
αchl,% |
90.0 |
100.0 |
100.0 |
90.0 |
||
As follows from the data of table 4, to achieve the 95-100% lead chloride sublimation degree the necessary temperature is 933.7‒1300°С and lgP should be from –0.48 to –1.75 (pressure of 0.33-0.0177 bar); for achievement of the 90.0-100% chloride sublimation degree for zinc respective parameters are T=1279.3-1500°C and lgP from –0.972 to –2 (pressure of 0.107-0,01 bar), for copper ‒ temperature of 1337.7-1500°С and lgP from –1.418 to –2 (pressure of 0.038-0.01 bar). Judging by the table data, at pressure of 0.01 bar the high copper chloride sublimation degree is possible at high temperatures (1337.7°С); for zinc it can be lowered to 1279.3°С and for lead ‒ to 933.7°С. At implementation of the sinter-chlorinating roasting of the slags the process temperature should not exceed the slag’s melting temperature (1200°С,12) that is its maximal value may be 1100-1150°С. For achievement of high αchl(Zn) and αchl(Cu) values at these temperatures the process pressure should be reduced.
The information about influence of the pressure reduction on αchl(Cu) at the lowered temperatures (1100-1150°С) is represented in table 5.
Table 5: Pressure effect on αchl(Cu) at the lowered temperatures.
| 1100°С | 3.6% (0.1 bar) | 14.3% (0.01 bar) | 54.8% ( 0.001 bar) | 100% (0.00032 bar) |
| 1150°С | 7.4% (0.1 bar) | 31,6% ( 0.01 bar) | 86.4% (0.001 bar) | 100% (0.00056 bar) |
It is evident, that at temperature of 1150°С and pressure of 0.001 bar αchl(Cu) exceeds 85%. At the same time, zinc and lead completely pass in a gaseous condition.
The Experiments Connected with Determination of the Possibility of Application of the Distilled Liquid for Extraction of Lead, Zinc and Copper from the Fuming Slags
The experiments connected with determination of the possibility of application of the distilled liquid for extraction of lead, zinc and copper from the fuming slags were carried out on a sintering plant.13 The charge mass was 300-350 g. Before the sintering roasting the slag and coal (5% of the slag mass) are grinded to the fraction <0.1 mm and pelletized using a solution of the distilled liquid. The granules in diameter of 1-1.2 cm were dried at 200°С during 30 min. The dry granules were loaded on a fire grate of an agglomeration cylinder (on which we preliminary placed a burnt limestone layer). Ignition of the granules is realized by means of a blowtorch. After the ignition (in 5-8 min) the layer wise burning of the charge with the simultaneous chloride sublimation of the metals occurred. Suction of the gases (including the chloride sublimation gases) is realized with the help of a smoke sucker. Vacuum under the fire-grate was controlled by a manometre. Duringthe ignition periodit was 0.006-0.0065 bar, and during thechloride sublimation period ‒ 0.01-0.015 bar. The process duration was 35-40 min. Temperature of the material in the layer was 1150-1180°С. The metals’ chloride sublimation degree was calculated under the formula:
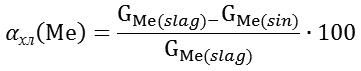
where GMe(slag) И GMe(sin) ‒mass of a metal in a slag and in a sinder after roasting, g. On the basis of the results of 3 experiments it was found, that the chloride sublimation degree of copper made 73-88%, zinc – 82-96% and lead ‒ 93-98%.
Conclusion
On the ground of the results of the experiments on application of the distilled liquid – the waste of soda manufacture – for extraction of lead, zinc and copper from the dump slags by chloride sublimation it is possible to draw the following conclusions:
At equilibrium conditions lead is extracted the most effectively from the slags by chloride sublimation , then zinc and copper: at pressure of 1 bar and temperature of 1500°С the chloride sublimation degree for lead makes 98.63%, for zinc – 64.45% and for copper – 21.91%;
To achieve the 95-100% lead chloride sublimation degree the needed temperature is 933.7‒1300°С and lgP should be from –0.48 to –1.75 (pressure of 0.33-0.0177 bar); for achievement of the 90.0-100% chloride sublimation degree for zinc respective parameters are T=1279.3-1500°C and lgP from –0.972 to –2 (pressure of 0.107-0,01 bar), for copper ‒ temperature of 1337.7-1500°С and lgP from –1.418 to –2 (pressure of 0.038-0.01 bar);
It was experimentally proved, that at the sinter-chlorinating roasting at temperature of 1150-11800С and pressure of 0.006-0.001 bar the chloride sublimation degree for lead makes 93-98%, for zinc ‒ 82-96%, for copper – 73-88%.
Author Contributions
All authors contributed in equal parts to the search and analysis of literature data, writing the text and designing the graphs. D. Pazylova is responsible for the coordination of work and correspondence with the journal.
Acknowledgments
The work was supported by projects of the Ministry of Education and Sciences of the Republic of Kazakhstan.
Conflicts of Interest
There is no conflict of interest.
References
- Lotosh, V.E. Processing of wastes of natural application.Yekaterinburg:University UST,(Russian), 2002, 463.
- Bakhonina, E.I.; Bikbulatov, I.H.; Bakiyev, A.Yu.; Daminev, R.R.; Nasyrov, R.R.; Oparina, F.R. A waterless calcium peroxide production way, RU2341449, 23.05.2007.
- Isayev, A.B.; Aliyev, Z.M.; Abdullaeva, N.A. A phosphorus-containing fertilizers production way, RU2398753, Publ. 10.09.2010.
- Mukhametov, А.А.; Voronin, A.V.; Sadykov, N.B.; Mustafin, A.G.; Mukhametov, A.A. An ammonium chloride production way, RU2495824,20.10.2013.
- Mikhailova, E.A;, Loboiko, A.Ya.; Molchanov V.I.; Panasenko, V.A. Theses of reports of the international conference “Cooperation for solving of the waste problem”(Russian), 2004, 177-178.
- Tkach G.A., Shaporev V.P., Titov. V.M. Soda manufacture according toa low-waste technology. Kharkov,(Russian), 1998.
- Bykovsky, N.A.; Kurbangaleyeva, L.R.; Daminev R.R. Basic research, (Russian),2012, 6(1), 209-213.
- Ghosh, P.K.; Mody, H.M.; Somani, R.S.; Maiti P.А.; Gandhi, M.R.; Bajaj, H.C.; Chunawala, J.R.; Upadhyay, S.C. Method of recycling of by-products for the production of soda ash and ammonium sulphate, US patent application 20150093309A1, 02.04.2015.
- Roine, A. Outokumpu HSС Chemistry for Windows. Chemical Reaction and Eguilibrium software with Extensive Thermochemical Database. Pori: Outokumpu Research OY, 2002.
- Akhnazarova, S.L.; Kafarov, V.V. Methods of experiment optimisation in the chemical industry. Moscow: Higher school, (Russian), 1978, 319.
- Ochkov,V.F. Mathcad-14 for students, engineers and designers.St.Petersburg: BHV-Peterburg, (Russian), 2007.
- Daribaev, Zh.Ye.; Shevko, V.M. Sinter-chlorinating roasting of concentration tailings and overburden rocks. Kentau, (Russian), 2004, 211.
- Shevko, V.M.; Karatayeva, G.Ye. Metallurgy of zinc and cadmium. Shymkent, (Russian), 2015, 350.

This work is licensed under a Creative Commons Attribution 4.0 International License.









